Orbital Pheochromocytoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
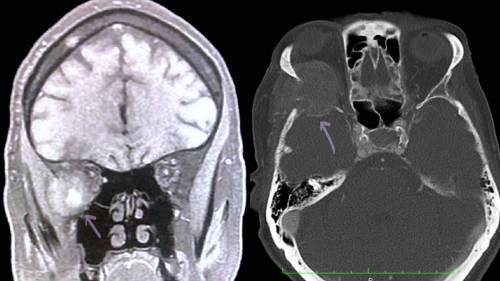
Pheochromocytomas are catecholamine secreting neuroendocrine tumors that originate from chromaffin cells in the adrenal medulla. Although most are benign, they can be malignant in approximately 10% of cases.[1] The classic presentation of patients with pheochromocytoma are headache, sweating and tachycardia. Rarely, pheochromocytoma metastases have been found in the orbital bone. When this occurs, patients may present with symptoms due to catecholamine release as well as mass effect on surrounding structures.[2]
Epidemiology
The incidence of pheochromocytoma is approximately 8 per 1,000,000 persons per year. It most commonly occurs during the 3rd-5th decade of life and is found in 0.1%-1.0% of patients with hypertension. It is the most common tumor of the adrenal medulla in adults.[3] Only 7 cases of orbital pheochromocytoma have been reported in the last 50 years. Five of those cases included metastasis of the orbital bones while only 2 cases involved metastasis of orbital bone and soft tissue.[4]
Pathophysiology of Disease
The adrenal medulla is made up of modified sympathetic postganglionic neurons and contains chromaffin cells with many secretory granules that store catecholamines.[4] Pheochromocytomas are tumors that originate from these cells. Paragangliomas also originate from chromaffin cells but are differentiated from pheochromocytomas as they originate at sites other than the adrenal medulla, such as the sympathetic ganglia of the carotid body and autonomic ganglia.[2] When metastasized, pheochromocytomas are commonly found in bone, liver, lymph node and lungs with bone being the most prevalent.[5][6][7] Local invasion of the omentum and kidneys also commonly occur. While primary paraganglioma of the orbit are not uncommon, metastatic orbital pheochromocytoma is exceedingly rare.[8]
Risk Factors
No known risk factors including environmental, dietary, or lifestyle have been linked to the development of pheochromocytoma or paraganglioma.[3]
Diagnosis
Clinical Presentation
Patients with orbital pheochromocytoma may present with symptoms consistent with increased catecholamine release. Onset of symptoms may occur with manipulation of the mass (through ocular massage or eye rubbing) causing a release of catecholamines into the blood supply to the associated area. However, malignant lesions tend to be less hormonally active than benign lesions, and orbital symptoms are predominantly due to mass effect.[2] General symptoms of pheochromocytoma are often paroxysmal and episodic in nature due to fluctuations in catecholamine levels but may also be sustained especially in children (70% of cases).[8] Many of these symptoms present with physical exam findings that increase suspicion and warrant further investigation. Associated signs and symptoms include:
Ocular Symptoms
- Orbital edema
- Appearance of Bulging eyes
- Ophthalmoplegia
- Blurry vision
- Double vision
- Facial numbness
General Symptoms
- Throbbing headache
- Diaphoresis
- Heart palpitations
- Abdominal pain
- Nausea
- Anxiety
- Tremor
- Weight loss due to increased metabolism
- Pallor
Signs
- Decreased visual acuity
- Diplopia
- Increased blood pressure
- Orthostatic hypotension (epinephrine-secreting pheochromocytomas)
- Tachycardia
- Exophthalmos
- Blepharoptosis
- New onset or worsened hyperglycemia
In some cases, patients may be asymptomatic and are only detected due to abnormal biochemical testing, or incidental findings on imaging. These cases are more common in patients with hereditary conditions and are found during routine testing.[8]
Ocular Examination
Ocular exam should include the following:
- Visual acuity
- Extraocular Muscle Movement
- Afferent Pupillary Defect
- Marginal reflex distance 1 measurements
- Hertel exophthalmometry measurements
Diagnostic procedures
The approach to diagnosing pheochromocytoma is three-fold. Biochemical testing detects excess catecholamine levels while imaging is necessary to locate and stage the extent of tumor burden. Finally, genetic testing may be indicated on an individual basis if germline mutations are suspected.[9][10][11]
Biochemical testing
Initial biochemical testing for pheochromocytoma should include measurements of plasma free metanephrine or urinary fractionated metanephrine. Significantly increased levels of both metanephrine and normetanephrine indicate a positive result and the diagnosis is highly likely. Alternatively, if either value is three times the upper limit of normal, it is also considered a positive result.[9][10][12]
Imaging
Computed Tomography (CT) versus Magnetic Resonance Imaging (MRI)
Imaging is indicated after excess catecholamine levels are confirmed in plasma or urine labs. While CT abdomen and pelvis with contrast is the modality of choice for primary pheochromocytoma, MRI is preferred to detect metastatic disease into the soft tissues, as CT imaging has low sensitivity for extra-adrenal tumors. On T1-weighted imaging, pheochromocytomas are either hypo or isointense whereas on T2 weighted imaging, the tumor appears as hyperintense.[8]
Functional Imaging
Functional imaging targets specific cell membrane transporters or vesicular catecholamine transport systems. This modality should be considered in patients with:
- Known or high risk of metastatic disease
- High clinical suspicion despite negative imaging on MRI or CT
- Incidental lesions found on imaging that are suspicious for paragangliomas inconclusive biochemical testing
- Assessment of regional extension or multifocality, and exclusion of metastases
MIBG scintigraphy (I-123 versus I-123 MIBG) and Positron Emission Tomography (PET)
In patients with known metastatic disease, positron emission tomography (PET) scans and meta-iodobenzylguanidine (MIBG) scintigraphy have significant utility to identify metabolically active tissue.
MIBG scintigraphy can be used with either I-123 or I-131 MIBG. I-123 is more sensitive than I-131 MIBG and produces better image quality, however it is more expensive. These studies use a norepinephrine precursor to localize the tumor.[8] They also provide functional information about the tumor and assesses the eligibility of I-131 MIBG (9,10,12). [9][10][12]
There are multiple options for PET scans each providing specific advantages. PET with [18F] fluorodihydroxyphenylalanine (FDOPA) is highly specific (95-100%) for pheochromocytoma. Another advantage is that it lacks uptake by healthy adrenal tissue and allows for better detection of multifocal pheochromocytomas and paragangliomas (multiple tumors in the same gland). Alternatively, PET with [18F] fluorodeoxyglucose (FDG) has much lower specificity than CT, MRI, and MIBG, but is useful in localizing metastatic pheochromocytomas and paragangliomas.[8]
Immunohistochemical Testing
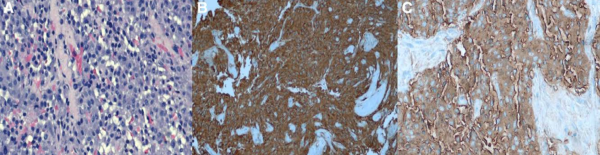
Immunohistochemical markers commonly used to diagnose pheochromocytoma include chromogranin A, neuron-specific enolase, protein gene product 9.5, CD56, glial fibrillary acidic protein and synaptophysin. Immunoreactivity of these markers in pheochromocytoma have been described as the following:
- Synaptophysin: strong, diffusely intracytoplasmic, and confined solely to neoplastic chromaffin cells. Cortical tissue showed no immunoreactivity with scattered capsular nerves strongly positive
- Chromogranin A: diffuse, granular, cytoplasmic immunoreactivity within neoplastic chromaffin cells, but spared the cortical cells.
- Protein gene product 9.5: diffuse cytoplasmic immunoreactivity in the neoplastic cells as well as staining occasional interstitial cells in the adrenal cortex and the capsular nerves.
- CD56 (N-CAM): strongly membranous in all of the neoplastic chromaffin cells. Cells in the zona reticularis and zona fasiculata had scattered, less intense membrane staining.
- Glial fibrillary acidic protein: universally negative immunoreactivity. [13]
Lesions that are S-100 positive are associated with familial syndromes but are not common in malignant lesions.[14] Malignant lesions are more commonly associated with HMB45.[2]
Histology
While biopsies are not indicated for diagnostic purposes, histologic examination of resected lesions will confirm the suspected diagnosis of metastatic pheochromocytoma postoperatively. The classic finding on histology is tight bundles, nests, and cords of neoplastic chromaffin cells. Necrosis of larger tumors is more common in malignant lesions. This necrosis has been postulated to be the cause of decreased hormone activity in malignant lesions.[2]
Genetic testing
Approximately, 35% of all pheochromocytomas and paragangliomas occur in the setting of hereditary syndromes. These include the following:
- Multiple Endocrine Neoplasia Type 2A and 2B
- Von-Hippel-Lindau syndrome
- Neurofibromatosis Type I
- Hereditary paraganglioma syndrome
- Germline fumarate hydratase mutation
- Germline transmembrane protein 127 mutation
- Germline MYC-associated factor X mutation
At least 12 pheochromocytoma and paraganglioma susceptibility genes have been identified, each associated with a hereditary syndrome and demonstrates varying penetrance for both primary and metastatic disease[15]. The most commonly associated gene with metastatic pheochromocytoma and paraganglioma is SDHB which is present in over 40% of cases reported at this time.[3]
Differential diagnosis
As pheochromocytoma produces symptoms of many other clinical conditions, it is known as the “great mimic." Differential diagnosis may include but is not limited to the following:
- Carcinoid syndrome
- Medullary thyroid carcinoma
- Adrenal carcinoma
- Hyperthyroidism (thyroid eye disease)
- Drug induced
- Monoamine oxidase inhibitors
- Sympathomimetics
- Clonidine withdrawal
- Recreational drugs (Cocaine, Methamphetamine, PCP, etc.) [16]
Management
Surgical removal is the mainstay of treatment. Patients with benign pheochromocytomas and paragangliomas have an equal survival rate when compared with the general population after resection. However, malignant pheochromocytomas and paragangliomas have a 5-year survival rate of <50% compared to age-matched controls.[17] In metastatic disease or when complete removal of the tumor is not possible, alternative treatments may include a combination of surgery, chemotherapy, radiation therapy, radiofrequency ablation, and cryoablation.[3]
Medical Management
Medical management is used to control symptoms of catecholamine release and optimize hemodynamic stability before other treatments are initiated. This includes the use of alpha-adrenergic blockers such as phenoxybenzamine usually in combination with a beta-adrenergic blocker, propranolol or atenolol. Alternatively, a calcium channel blocker combined with an alpha-adrenergic blocker may be used. If blood pressure remains refractory to initial medical management, a catecholamine synthesis inhibitor such as metyrosine, may be added.[17]
Chemotherapy
Chemotherapy has not been shown to be effective in increasing survival rates in patients with metastatic pheochromocytoma. However, it has been shown to be effective for symptom palliation. The best-established chemotherapy regimen is the Averbuch protocol (cyclophosphamide, vincristine, and dacarbazine). [17][18]
This regimen has been reported to be well tolerated without any significant side effects. Side effects that have been reported include myelosuppression, peripheral neuropathy, gastrointestinal toxicity, and hypotension. Massive spontaneous catecholamine release can occur after drug administration leading to more serious adverse effects such as tachyphylaxis and cardiogenic shock. It is recommended that the first cycle of chemotherapy be administered with close monitoring, possibly in collaboration with an endocrinologist.[17]
Radionucleotide therapy
Approximately 60% of metastatic pheochromocytomas are positive for MIGB uptake in which Iodine-131 MIBG radiation therapy has been used as a palliative treatment.[3] Hematologic toxicity was the most frequently reported side effect with neutropenia occurring in 87% of treatments and thrombocytopenia occurring in 83% of treatments. One meta-analysis showed stable disease following I-131 MIBG therapy in 52% of cases and a partial hormone response (reduction of catecholamine secretion) in 43% of cases.[19]
Surgical Management
Surgery has been suggested as a viable option for reduction of tumor burden in patients with orbital pheochromocytoma.[2] However, extensive preoperative, intraoperative, and postoperative precautions must be taken as manipulation of the tumor may produce catecholamine release causing life threatening complications including hypertensive crisis, arrythmia, pulmonary edema, and myocardial infarction.
It is imperative that adrenergic blockade should be initiated 1-2 weeks preoperatively to prevent associated symptoms from the release of catecholamines during surgical manipulation. Phenoxybenzamine is the drug of choice, however, selective alpha-1-antagonists such as terazosin, prazosin, and doxazosin may be used as alternative options. If increased blood pressure or tachycardia are refractory to proper alpha-adrenergic blockade, a beta-adrenergic blocker may be added. However, a beta blocker must never be initiated before an alpha blocker due to unopposed alpha-adrenergic vasoconstriction.
Additionally, salt and fluid intake should also be increased while taking alpha antagonists as increased fluid volume reduces orthostatic hypotension and subsequent catecholamine release both preoperatively and postoperatively and helps to maintain hemodynamic stability intraoperatively.[3] Blood pressure, heart rate and blood glucose levels should be monitored preoperatively, intraoperatively and in the immediate postoperative period.[9] Intraoperatively, continuous blood pressure monitoring occurs through intra-arterial catheters and assists in managing blood pressure fluctuations.[8]
Follow-up
Clinical guidelines recommend obtaining plasma or urine metanephrine levels to detect persistent disease as well as lifelong annual biochemical testing to assess for recurrence.
Prognosis
Patients with benign disease should experience an overall survival rate similar to that of the general population. However, approximately 6% to 17% of individuals with benign disease will have recurrence usually within 5-15 years after initial surgery. Approximately 15-25% of patients with recurrence develop metastatic disease. The 5-year survival in patients with metastatic disease ranges from 50% to 70%. Carriers of SDHB variants have an increased risk of developing metastatic disease of roughly 25% to 50%.[3]
References
- ↑ Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo J. Harrison’s Principles of Internal Medicine. 20th ed. New York: McGraw-Hill Education; 2015.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Rider AJ, Walsh A, Sollenberger EL, Dryden SC, DeAngelis KD, Weir AB, et al. Orbital Pheochromocytoma Metastasis in 2 Patients With Known Pheochromocytoma. Ophthalmic Plast Reconstr Surg. 2019;35(6):e131–4.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 PDQ Adult Treatment Editorial Board. Pheochromocytoma and Paraganglioma Treatment (PDQ®): Health Professional Version. 2022.
- ↑ 4.0 4.1 Rider AJ, Walsh A, Sollenberger EL, Dryden SC, DeAngelis KD, Weir AB, et al. Orbital Pheochromocytoma Metastasis in 2 Patients With Known Pheochromocytoma. Ophthalmic Plast Reconstr Surg. 2019;35(6):e131–4.
- ↑ Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983 Dec;58(12):802–4.
- ↑ Lynn MD, Braunstein EM, Wahl RL, Shapiro B, Gross MD, Rabbani R. Bone metastases in pheochromocytoma: comparative studies of efficacy of imaging. Radiology. 1986 Sep;160(3):701–6.
- ↑ Drasin H. Treatment of malignant pheochromocytoma. West J Med. 1978 Feb;128(2):106–11.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Rider AJ, Walsh A, Sollenberger EL, Dryden SC, DeAngelis KD, Weir AB, et al. Orbital Pheochromocytoma Metastasis in 2 Patients With Known Pheochromocytoma. Ophthalmic Plast Reconstr Surg. 2019;35(6):e131–4.
- ↑ 9.0 9.1 9.2 9.3 Lenders JWM, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SKG, Murad MH, et al. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014 Jun 1;99(6):1915–42.
- ↑ 10.0 10.1 10.2 Chen H, Sippel RS, O’Dorisio MS, Vinik AI, Lloyd R V., Pacak K. The North American Neuroendocrine Tumor Society Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors. Pancreas. 2010 Aug;39(6):775–83.
- ↑ Fassnacht M, Assie G, Baudin E, Eisenhofer G, de la Fouchardiere C, Haak HR, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2020 Nov;31(11):1476–90.
- ↑ 12.0 12.1 Neumann HPH, Young WF, Eng C. Pheochromocytoma and Paraganglioma. New England Journal of Medicine. 2019 Aug 8;381(6):552–65.
- ↑ Miller AD, Masek-Hammerman K, Dalecki K, Mansfield KG, Westmoreland S V. Histologic and Immunohistochemical Characterization of Pheochromocytoma in 6 Cotton-top Tamarins ( Saguinus oedipus ). Vet Pathol. 2009 Nov 15;46(6):1221–9.
- ↑ Tatić S, Havelka M, Paunović I, Bozić V, Diklic A, Brasanac D, et al. [Pheochromocytoma--pathohistologic and immunohistochemical aspects]. Srp Arh Celok Lek. 2002 Jul;130 Suppl 2:7–13.
- ↑ Fishbein L. Pheochromocytoma/Paraganglioma: Is This a Genetic Disorder? Curr Cardiol Rep. 2019 Sep 31;21(9):104.
- ↑ Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. The Lancet. 2005 Aug;366(9486):665–75.
- ↑ 17.0 17.1 17.2 17.3 Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine. Cancer. 2008 Oct 15;113(8):2020–8.
- ↑ Averbuch SD. Malignant Pheochromocytoma: Effective Treatment with a Combination of Cyclophosphamide, Vincristine, and Dacarbazine. Ann Intern Med. 1988 Aug 15;109(4):267.
- ↑ Van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EPM. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014 Apr;80(4):487–501.

