Orbital Mesenchymal Chondrosarcoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Orbital Mesenchymal Chondrosarcoma is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
ICD-10:
C41.0 Malignant neoplasm of bones of skull and face
C69.60 Malignant neoplasm of unspecified orbit
C69.61 Malignant neoplasm of right orbit
C69.62 Malignant neoplasm of left orbit
Disease
Orbital mesenchymal chondrosarcoma is a malignant neoplasm composed of small round cells and mature cartilage (Hererra 2012).[1] Although rare, the orbit is the third most common site of extra-skeletal mesenchymal chondrosarcoma (Alkatan 2018).[2] The tumor can form in soft tissues within the orbit or within the orbital bones (Duane 1985).[3]
History
Orbital mesenchymal chondrosarcoma was first described in 1959 by Lichtenstein and Bernstein (Hererra 2012).[1] In 1969, Trzcinska-Dabrowska and colleagues identified an orbital mesenchymal chondrosarcoma attached to periorbital bone. In 1971, the first case of soft tissue orbital mesenchymal chondrosarcoma, which was closely attached to extraocular muscles, was published by Cardenas-Ramirez (Duke-Elder 1974).[4] The lesion presented in a 38 year old male and was the 19th case of orbital mesenchymal chrondrosarcoma published throughout the world (Cárdenas-Ramírez 1971).[5] The first case of congenital orbital mesenchymal chondorsarcoma was published in 2014, discovered as a papillomatous lesion of the tarsal conjunctiva in a 5 month old girl (Tuncer 2004).[6] As of 2018, nearly 30 cases of orbital mesenchymal chondrosarcoma have been reported in English literature (Alkatan 2018).[2]
Definitions
The term orbital mesenchymal chondrosarcoma is used to describe a malignant neoplasm composed of undifferentiated mesenchymal cells and mature cartilage.
Epidemiology
Mesenchymal chondrosarcoma accounts for 2-3% of all chondrosarcomas. A third of mesenchymal chondrosarcomas occur extra-skeletally, and of these, the orbit is the third most common site of this neoplasm after the meninges and lower extremities (Alkatan 2018).[2] Ten percent of extra-skeletal mesenchymal chondrosarcomas occur in the head and neck, commonly in the maxilla and mandible (Hererra 2012).[1]
Patients with orbital mesenchymal chondrosarcoma tend to be younger than patients with chondrosarcoma in other sites (Hererra 2012).[1] It most commonly occurs in the second or third decades, however, outliers include several cases in children (Alkatan 2018)[2] and one in an elderly, 84 year old female (Shimo-Oku 1980).[7] Orbital mesenchymal chondrosarcoma is thought to be more common in women (Alkatan 2018, Murphey 2003).[2][8]
Classification
Mesenchymal chondrosarcoma can be classified into skeletal and extra-skeletal neoplasms. Of those occurring extra-skeletally, they can be further classified into those occurring in skeletal muscle and soft tissue versus those occurring in the central nervous system and spinal cord (Arora 2018).[9] Among orbital tumors themselves, no additional classification has been described.
Stages
Orbital mesenchymal chondrosarcoma may be staged according to the TNM system as described by the American Joint Committee on Cancer, based on the tumor size, involvement of lymph nodes, and presence of metastases.
Pathophysiology
The undifferentiated mesenchymal cells and the mature cartilage found in this neoplasm originate from cartilage-forming primitive mesenchyme. The histology and growth pattern of these neoplasms have been found to resemble the growth pattern of fetal cartilage, which is centripetal in nature. Further, these neoplasms have also been shown to have features resembling the early condensational phase of cartilaginous differentiation. The gene, Sox9, which regulates chondrogenesis, has been found to be expressed in 95.5% of extraskeletal mesenchymal chondrosarcomas (Arora 2018).[9]
In fact, various genetic associations to mesenchymal chondrosarcoma have been found. A study by Meijer and colleagues found that 70% of mesenchymal chondrosarcomas studied were associated with an expression of p53 and IDH1, which have important roles in retinoblastoma pathways as well (Meijer 2012).[10] Further, in two cases of mesenchymal chondrosarcoma (one skeletal and another extra-skeletal), a 13:21 Robertsonian translocation (when a certain type of chromosome becomes attached to another) has been identified. Other isolated cases have been associated with trisomy 8 and a reciprocal translocation of (11;22)(q24;q12). The World Health Organization reports that the gene fusion, HEY1-NCOA2, may be marker of potential clinical utility in cases of extra-skeletal mesenchymal chondrosarcoma. Additionally, a fusion of the IRF2BP2 gene and the transcription factor CDX1 gene has also been identified in cases of extra-skeletal mesenchymal chondrosarcoma (Arora 2018).[9]
Diagnosis
History
Patients with mesenchymal chondrosarcoma of the orbit tend to be females in their second or third decades, but the tumor can present in any age or gender. In their history, patients may report a gradual loss of vision or diplopia. They may also complain of proptosis, ptosis, or pain (Hererra 2012).[1] Patients with secondary metastases may have additional systemic complaints. Additionally, patients may also have a history of bilateral retinoblastoma; children with such a history are at risk for orbital mesenchymal chondrosarcoma and other malignant mesenchymal tumors, even if they were not treated with radiation (Foster 2018).[11]
Physical examination
The normal thorough ophthalmologic exam should be performed with attention to visual acuity, pupils, and color vision for possible optic neuropathy. The eyelids and ocular adnexa should be examined for masses or changes in position, like ptosis or retraction. Extraocular movements, exophthalmos, and lagophthalmos can assess for extraocular muscle restriction, proptosis, or orbital mass. The optic nerve head can assess for optic nerve edema or atrophy.
Signs
Initial signs of orbital mesenchymal chondrosarcoma can be proptosis, followed by reduced vision (Garrity 2007).[12] Other signs seen on physical exam include eyelid edema, conjunctival mass, lagophthalmos, ptosis, hypoglobus, or a frozen globe. Patients may have a relative afferent pupillary defect. Optic nerve edema can be seen in advanced cases (Alkatan 2018).[2] Metastases, while rare, may also be found (Garrity 2007).[12] These orbital tumors tend to metastasize to the lungs (Duane 1985).[3]
Symptoms
Patients may present with a variety of symptoms including pain, diplopia, worsening vision, or conjunctival lesion. Patients may also report pain with extra-ocular movements and tearing (Alkatan 2018).[2] Cases of intracranial extension have been reported, in which case, patients may report headache (Sharma 2015).[13]
Clinical diagnosis
The diagnosis is usually made anywhere from 2 weeks to 19 months after symptom onset, with a mean of 6 months to time until diagnosis (Alkatan 2018).[2] While the signs and symptoms of the neoplasm are helpful, more essential are radiologic imaging, immunohistochemistry, and histopathology. The tumor is diagnosed primarily on the basis of histologic morphology due to its nonspecific immunoprofile and a general lack of availability of molecular diagnostics (Arora 2018).[9]
Laboratory tests
While serology has not been reported to aid in the diagnosis, the various ancillary tests as described below (pathologic examination, radiologic imaging, and immunohistochemistry) are of tremendous value.
Histopathology
Grossly, the tumor is usually a lobulated, well-circumscribed mass with a thin, fibrous capsule (Arora 2018).[9] It may be focally calcified, cartilaginous, or vascular. Its consistency may be soft or firm. The color can be white, grey, tan, or red, and the cartilaginous areas may be blue. Necrosis and hemorrhage are commonly seen on gross examination (Alkatan 2018, Duane 1985).[2][3]
Microscopically, the tumor has a biphasic pattern of undifferentiated mesenchymal cells and mature cartilage. The undifferentiated mesenchymal cells have round or elongated hyperchromatic nuclei and scanty cytoplasm. These cells are usually arranged in sheets or clusters. The cartilaginous areas may show loosely arranged mature hyaline cartilage, coarse calcification, or endochondral ossification with bone formation (Alkatan 2018).[2] The cartilaginous areas are usually well-circumscribed; they can, rarely, have indistinct borders and merge with the mesenchymal cells (Arora 2018).[9] Type 2 collagen is found in the extracellular matrix both in chondroid islands and non-chondroid areas. Pathology may also reveal a hemangiopericytic pattern, with delicate vascular channels among the mesenchymal cells (Garrity 2007).[12]
Imaging
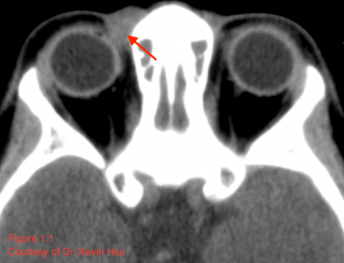
CT usually reveals a well-defined, heterogeneously enhancing mass with areas of fine and coarse calcification (Font 2009).[14] Such radiologic appearance is considered characteristic, and internal calcifications in particular should raise concern for possible mesenchymal chondrosarcoma (Angotti-neto 2006, Koeller 1999).[15][16] However, not all tumors will demonstrate such calcification (Lu 2009).[17] The lesion tends to have the same attenuation as muscle (Murphey 2003)[8], and generally, the radiopaque areas correlate with areas of high-grade sarcoma and hemangiopericytoma while radiolucent portions correspond to the areas of chondrosarcoma (Font 2009).[14] Figure 1.1 illustrates a case of orbital mesenchymal chondrosarcoma in which the CT revealed a homogenous isodense mass with the same attenuation as muscle and no areas of calcification. Although the lesion appeared non-specific on CT, the diagnosis was confirmed by histopathology after resection.
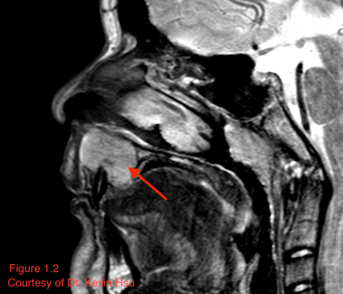
MRI can also reveal mild to moderate enhancement and calcified areas of the mass (Alkatan 2018).[2] The non-calcified portions of the tumor have a signal intensity equal or lower to gray matter on T1 weighted images, while being isointense to grey matter on T2-weighted images (Shinaver 1997).[18] In fact, the focal low-intensity areas surrounded by high-intensity areas on T2-weighted MRI have been thought to be characteristic of this neoplasm (Arora 2018).[9] MR may also reveal necrotic areas, as well as serpentine high-flow vessels which correlate to the areas of hemangiopericytoma (Murphey 2003).[8] Figure 1.2 illustrates a case of maxillary chondrosarcoma in which the lesion appeared isointense to hyperintense on T2, with a few foci of hypointensity possibly representing chondroid matrix mineralization.
Imaging may show the tumor in its more common location in the posterior retrobulbar space. The tumor has been reported to occur both within the muscle cone (Alktan 2018, Lu 2009)[2][17], as well as extraconally (Patel 2012).[19] Bony destruction, periosteal reaction, optic nerve sheath invasion, or muscle infiltration are rare but can occur (Garrity 2007, Murphey 2003).[12][8] However, the orbital bone may be widened from infiltration of the slowly growing lesion. Overall, CT and MRI are used in combination they can help identify mesenchymal chondrosarcoma from other similar lesions (Shinaver 1997).[18]
Immunohistochemistry
Immunohistochemistry can be extremely useful in identifying mesenchymal chondrosarcoma. These neoplasms show S-100 protein positivity in the cartilaginous areas and may also show scant positivity in the mesenchymal regions (Arora 2018).[9] The cellular regions tend to be positive for CD99 and vimentin (Alkatan 2018)[2] and may also be positive for neuron-specific enolase an Leu-7. Sox9 may be positive in both the mesenchymal and cartilaginous areas. The neoplasm stains positive for INI-1, which can help exclude epithelioid sarcoma, myxoid chondrosarcoma, or myoepithelial carcinoma (Arora 2018).[9]
Immunohistochemistry is usually negative for vimentin, actin, and cytokeratin (Hererra 2012).[1] Extra-skeletal chondrosarcomas are also negative for FLI-1, which can help in excluding the diagnosis when considering Ewing sarcoma. Nonetheless, the immunoprofile is not specific enough for the diagnosis of this neoplasm, and the morphology as seen on pathology continues to be the basis for diagnosis (Arora 2018).[9]
Differential diagnosis
Differential diagnoses for the presentation of an orbital mesenchymal chondrosarcoma is broad. It includes myxochondrosarcoma, osteogenic sarcoma, osteochondroma, lymphoma, neuroblastoma, synovial cell sarcoma, neurofibroma, meningioma, schwannoma, sclerosing hemangioma, rhabdomyosarcoma, fibrous histiocytoma, and solitary fibrous tumor (Hererra 2012, Alkatan 2018).[1][2] However, because mesenchymal chondrosarcomas of the orbit are rare, one’s level of suspicion must be high in order to diagnose early.
Management
Surgical therapy
Surgery is the mainstay of treatment. When the tumor was initially identified, historically, exenteration was the primary form of removal (Garrity 2007)[12], and it may still occasionally be an option (Hanakita 2012).[20] However, resection with wide margins has now been found to be effective (Alkatan 2018).[2] As the tumor is usually located in the posterior orbit adjacent to the optic nerve, it is helpful to have both an oculoplastic surgeon and neurosurgeon present to remove the malignancy via coronal craniotomy. This allows for a wide view to allow complete excision and adequate control of bleeding during surgery (Garrity 2007).[12]
Medical and radiation oncology therapy
Chemotherapy and radiation are other treatment modalities, which may be used when the tumor cannot be resected or appears aggressive on histology (Alkatan 2018).[2] Radiation can be used pre-operatively to reduce tumor bulk. Additionally, patients who receive post-operative radiation tend to have increased survival. However, this has not been shown to be statistically significant and some believe that these neoplasms may be resistant to radiation (Hererra 2012).[1]
Chemotherapy is used as an adjuvant for aggressive tumors, including high grade lesions, those with rapid local recurrence, or those with potential metastases. Mesenchymal chondrosarcoma is often treated with the same regimen as Ewing’s sarcoma. A case report by Herrera et al noted that their patient with primary orbital mesenchymal chondrosarcoma was treated as such, and that the standard regimen includes vincristine, dactinomycin, cyclophosphamide and doxorubicin. Another chemotherapy option is ifosfamide with etoposide, which has shown an improved 5 year disease-free survival. Newer chemotherapy agents may target molecular pathways involved, including the phosphoinositide-3 kinase and MEK-extracellular signal-regulated kinase (ERK) signaling, as well as proliferator-activated receptor-gamma (PPAR-c) activity (Hererra 2012).[1] Overall, it is thought that multimodal therapy may lead to long-term survival and many patients now are treated as such with improved outcomes in recent years (Tuncer 2004).[6]
Disease monitoring
The prognosis for mesenchymal chondrosarcoma is poor and unpredictable (Duane 1985).[3] The all site survival rate is 55% at 5 years (Hererra 2012).[1] It has a long term survival of approximately 30% (Jacobs 1994).[21] This may be partly due to the fact that it has a tendency to recur late and can have delayed distant metastasis (Odashiro 2009).[22] However, orbital lesions have a higher survival rate than lesions in other sites. Indeed some patients may have complete tumor response and long term survival. Nonetheless, the tumor can recur or metastasize even 20 years after treatment, thus long-term monitoring in addition to early detection is essential (Hererra 2012, Alkatan 2018).[1][2]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Herrera A., Ortega C., Reyes G., Alvarez M.A., Tellez D., Primary Orbital Mesenchymal Chondrosarcoma: Case Report and Review of the Literature.” Case Reports in Medicine. 2012, 292147.
- ↑ Jump up to: 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 Alkatan, H. M., Eberhart, C. G., Alshomar, K. M., Elkhamary, S. M., & Maktabi, A. Primary mesenchymal chondrosarcoma of the orbit: Histopathological report of 3 pediatric cases. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society, 2018; 32(1): 69–74.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Duane T. D. & Jaeger E.A. (Eds.) (1985). Clinical Ophthalmology: Volume 2. Philadelphia, PA: Harper & Row Publishers.
- ↑ Duke-Elder S.S. & MacFaul P.A. (1974). The Ocular Adnexa: Volume XIII. London: Henry Kimpton.
- ↑ Cárdenas-Ramírez L., Albores-Saavedra J., de Buen S. Mesenchymal Chondrosarcoma of the Orbit Report of the First Case in Orbital Location. Arch Ophthalmol. 1971; 86(4): 410–413.
- ↑ Jump up to: 6.0 6.1 Tuncer S., Kebudi R., Peksayar G. Congenital mesenchymal chondrosarcoma of the orbit. Case report and review of literature. Ophthalmology. 2004; 111: 1016–1022.
- ↑ shimo-oku m., okamoto n., ogita y., sashikata t. a case of mesenchymal chondrosarcoma of the orbit. acta ophthalmol. 1980; 58: 831–840.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Murphey M.D. et al. (2003). Imaging of Primary Chondrosarcoma: Radiologic-Pathologic Correlation. Radiographics, 23(5).
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 9.9 Arora K. and Riddle N.D. Extraskeletal Mesenchymal Chondrosarcoma. Archives of Pathology & Laboratory Medicine. 2018; 142 (11): 1421-1424.
- ↑ Meijer D, et al. Genetic characterization of mesenchymal, clear cell, and dedifferentiated chondrosarcoma. Genes Chromosomes Cancer, 2012; 51: 899–909.
- ↑ Foster, J.A., Carter K.D., Durairaj V. D., Hartstein M.E., Kavanagh M.C., Korn B.S., Nelson, C.C. (Eds.) (2018). 2018-2019 Basic and Clinical Science Course: Orbit, Eyelids, and Lacrimal System. USA: American Academy of Ophthalmology.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 Garrity J.A. and Henderson J.W. (2007). Henderson’s Orbital Tumors: 4th edition. Rochester, MN: Lippincott Williams & Wilkins.
- ↑ sharma s, roy s, khan i, pal sk, majumdar a. orbito-cranial mesenchymal chondrosarcoma in a young female: a rare case report. clin cancer investig j. 2015; 4: 416-418.
- ↑ Jump up to: 14.0 14.1 Font R.L., Ray R., Mazow M.L., Del valle M. Mesenchymal chondrosarcoma of the orbit: a unique radiologic-pathologic correlation. Ophthal Plast Reconstr Surg. 2009; 25(3): 219–222.
- ↑ Angotti-neto H., Cunha L.P., Oliveira A.V., Monteiro M.L. Mesenchymal chondrosarcoma of the orbit. Ophthal Plast Reconstr Surg. 2006; 22(5): 378–382.
- ↑ Koeller K.K. Mesenchymal chondrosarcoma and simulating lesions of the orbit. Radiol Clin North Am, 1999; 37(1): 203-217.
- ↑ Jump up to: 17.0 17.1 Lu, H., Yang Y., Luo Q., He W. Mesenchymal chondrosarcoma of the orbit: report of a case and review of the literature. International Journal of Ophthalmology, 2009; 2(2): 185-188.
- ↑ Patel R. and Mukherjee B. (2012). Mesenchymal chondrosarcoma of the orbit. Orbit, 31(2): 126-128.
- ↑ Hanakita S., Kawai K., Shibahara J., Kawahara N., Saito N. Mesenchymal chondrosarcoma of the orbit–case report. Neurol Med Chir (Tokyo) 2012; 52(10): 747–750.
- ↑ Jacobs J.L., Merriam J.C., Chadburn A. Mesenchymal chondrosarcoma of the orbit. Report of three new cases and review of the literature. Cancer, 1994; 73: 399–405.
- ↑ Odashiro A.N., Leite L.V., Oliveira R.S. Primary orbital mesenchymal chondrosarcoma: a case report and literature review. Int Ophthalmol. 2009; 29(3): 173–177.