Orbital Adipose Tissue
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Orbital Adipose Anatomy
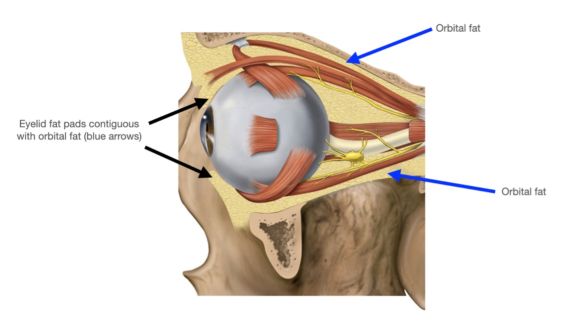
Orbital adipose tissue (fat) is located within the orbit surrounding the globe, optic nerve, and extraocular muscles. The orbital adipose tissue is a unique collection of white fat distinct from the subcutaneous adipose tissue elsewhere in the body. White adipose tissue stores excess energy as triglycerides, whereas brown adipose tissue dissipates energy through thermogenesis.[1] While systemic adipocytes range from 40 to 150 micrometers in size[2], orbital adipocytes studied under light microscopy have been found to be 50% the size of systemic adipocytes[3]. An analysis of orbital adipose tissue by microscopy and computer software modeling found smaller adipocyte cell diameter and a higher concentration of connective tissue elements such as blood vessels and elastic tissues when compared to samples of systemic adipose tissue[4].
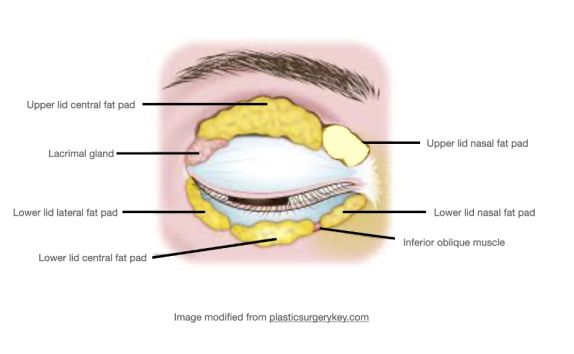
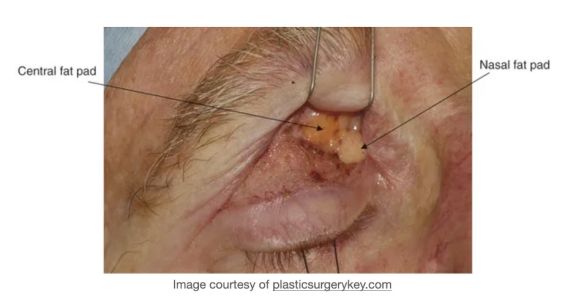
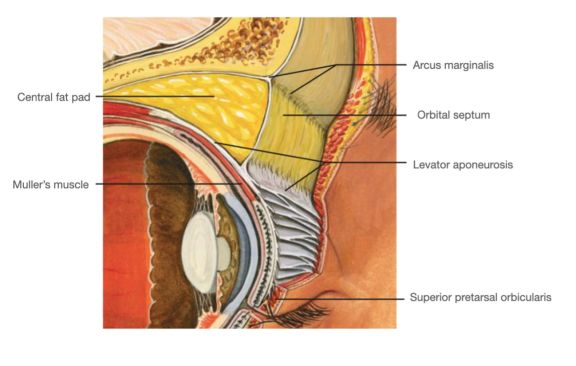
The upper eyelid orbital fat consists of two fat pads: the nasal, or medial fat pad and the central, or preaponeurotic, fat pad (Figure 1B, 1C). The lower eyelid contains three fat pads (though the medial and central pad are connected but bisected by the inferior oblique muscle). The upper eyelid nasal fat pad is contiguous with the orbital fat (Figure 1A). The central fat pad located between the levator muscle/aponeurosis, the orbital septum, and the orbital roof periosteum, and is contiguous with deeper orbital fat posteriorly as well (Figure 1D)[5] [6].
The upper lid nasal fat pad is white in color while the central fat pad is yellow. The richer yellow hue of the upper lid central fat pad can be attributed to its higher concentration of carotenoids such as beta-carotene and lutein [7]. The white nasal fat pad contains more fibrous tissue and is derived from neuroectoderm, while the yellow central fat pad is sparsely vascularized and derived from mesoderm. Carotenoids are known antioxidants, though their role in this location has not yet been defined[7]. The difference in color between these two upper lid fat pads stems from their different carotenoid contents, vascularity, and embryological origins. The vast majority of research is focused on the upper eyelid fat pads, possibly owing to its abundance and accessibility during routine upper eyelid blepharoplasty.
Orbital Adipose Embryology
Adipose tissue, along with other connective tissues such as cartilage, bone, blood, and lymph, develop from a loosely organized embryologic framework called mesenchyme. While most mesenchymal tissues are derived from mesoderm, some head and neck mesenchymal tissues are derived from neuroectoderm. Most adipose tissue throughout the body is derived from mesoderm, the middle embryologic germ layer. Orbital adipose tissue is unique in that it has both mesodermal and neuroectodermal origins. Specifically, the intraconal fat and nasal fat pads of the upper and lower lids are derived from neuroectoderm while the upper and lower eyelid central fat pads and lower eyelid lateral fat pad are derived from mesoderm[8] [9]. Interestingly, the nasal and central lower eyelid fat pads are contiguous and separated only by the inferior oblique muscle. When the fat pads are dissected intra-operatively from the inferior oblique muscle, they can be slid back and forth underneath the muscle, a movement termed “inverse shoe-shine sign”[10]. Post-septal extraconal orbital fat begins developing at week 14,[8], while the central fat pad develops at week 16 and the nasal fat pad develops at week 18[5].
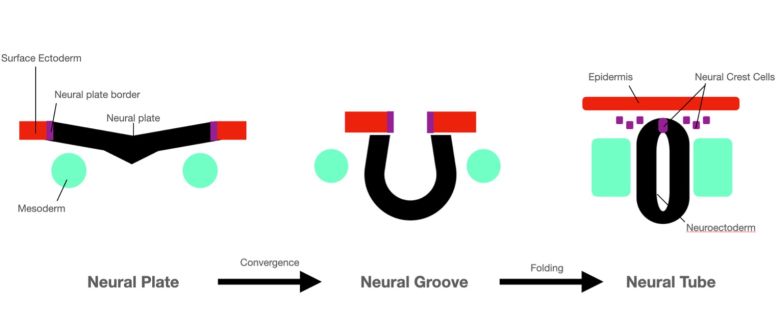
Neurulation is the formation process of the neural tube, from which the neural crest cells derive (Figure 2)[11]. This process begins at week 4 of gestation. The neural plate folds to form the neural groove, and as the opposing edges of the neural plate fuse, the neural tube is formed. The dorsal ectoderm of the neural plate becomes the neuroectoderm, located on the inner lining of the neural tube[11].
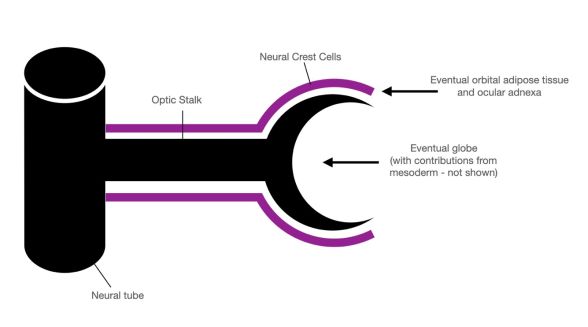
Neuroectoderm is the precursor to the central nervous system. Specialized migratory neural crest cells located at the crest of the ectodermal waves fuse during neural tube formation. These cells migrate to various destinations throughout the head and body to differentiate into a variety of cell types (Figure 3)[8].
Once the neural crest cells have migrated to their destination, these anlagen interact with mesoderm to differentiate into adipocytes of the intraconal orbital adipose tissue and nasal fat pad, as well as the levator aponeurosis, Muller’s muscle, tarsal plate, and eyelid melanocytes[8] [12].
Stem Cells: An Overview
Stem cells are self-renewing cells which can differentiate into various cell types, depending on their lineage. Stem cells can be categorized as pluripotent or multipotent. Pluripotent stem cells can differentiate into any cell type found in the body. They can differentiate into any cell of the three embryonic germ layers: ectoderm, mesoderm, and endoderm[13].
Two types of pluripotent cells are embryonic and induced pluripotent cells. Human embryonic stem cells remain a controversial research topic, while induced pluripotent cells provide an alternative avenue for pluripotent stem cell research without relying on human embryonal cells.
Multipotent stem cells can only differentiate into the cell types found in the tissue of origin. Their role in the adult human body is that of repair and maintenance. Three types of multipotent stem cells are hematopoietic stem cells, neural stem cells, and mesenchymal stem cells. Mesenchymal stem cells are adult stem cells with self-renewal capabilities which differentiate into various connective tissues including adipocytes, chondrocytes, and osteocytes[13].
Orbital Adipose Stem Cells
The isolation and characterization of orbital adipose stem cells (OASC) was first reported in 2009 (Korn). Since then, there have been multiple studies describing the similarities and differences between OASC and other types of adult human stem cells. Attention has mainly been focused on upper eyelid fat pads as these tissues are readily accessible and commonly removed during routine upper eyelid blepharoplasty.
Unique methods to optimize isolation of OASC have been described[9]. Systemic (non-orbital) adipose stem cells (ASC) can be isolated in vitro by first digesting adipose tissue with collagenase followed by centrifugation, which separates the mixture into a stromal vascular fraction (SVF) and retained cells (RC). While conventional methods of isolating ASC solely utilize the SVF, when this method is applied to OASC it leaves behind significant cell populations found in the RC after centrifugation due to the small size and basement membrane composition of OASC. Some adipocyte progenitor cells become entangled in the type IV collagen basement membrane of mature adipocytes in the RC, since collagenase does not dissolve type IV collagen. Thus, OASC should be isolated utilizing both the SVF and the RC[9].
Mesoderm-derived OASC share over 260 surface markers with bone marrow stem cells (Table 1)[14] [15]. Like other mesenchymal stem cells, OASC express characteristic markers CD90 and CD105[16] [17]. When comparing OASC to other mesenchymal stem cells such as bone marrow stem cells, OASC were found to have similar levels of a wide variety of transcription factors, proliferation and signaling genes, and other surface markers. Markers present in neuroectoderm-derived OASC include Nestin, beta-tubulin III, and Neurod1.
| Surface Marker | Gene | Role | Nasal and Central OASC Expression |
|---|---|---|---|
| CD34 [16] | CD34 gene | Transmembrane glycoprotein which functions in cell-cell adhesions on leukocytes; hematopoietic stem cell marker | Expressed 2-fold as much in nasal OASC |
| CD73[17] | NT5E | Widely expressed cell surface enzyme which functions as co-signaling molecule on T-lymphocytes; implicated in cancer | Similar expression |
| CD81[17] | CD81 gene | Widely expressed cell surface glycoprotein which promotes muscle cell fusion, B and T cell activation; implicated in cancer | Similar expression |
| CD90[16][17] | THY-1 | Cell surface glycoprotein which functions in inflammation by synthesizing and releasing growth factors, cytokines, and extracellular matrix components; adult mesenchymal stem cell marker | Similar expression |
| CD105[16][17] | ENG | Transmembrane glycoprotein receptor for transforming growth factor beta; adult mesenchymal stem cell marker | Similar expression |
| CD253[17] | TNFSF1-0 | Cytokine of tumor necrosis factor family which preferentially induces apoptosis in tumor cells but does not kill healthy cells | Similar expression |
| Nestin[16][17] | NES | Intermediate filament expressed in CNS multipotent stem cells; neural stem cell marker | Greater expression in nasal OASC |
| Beta-tubulin III[16][17] | TUBB3 | Core protein family which assembles to form microtubules | Greater expression in nasal OASC |
| Neurod1[17] | NEUROD | Transcription factor which regulates insulin expression; implicated in diabetes | Similar expression between nasal and central OASC |
| SOX2[17] | SOX2 gene | Transcription factor which confers self-renewal capability; mutations implicated in optic nerve hypoplasia with syndromic microphthalmia | Greater expression in OASC than bone marrow stem cells, similar in nasal and central OASC |
| Nanog[17] | NANOG | Transcription factor which confers self-renewal capability, required for pluripotency in embryonic stem cells and induced pluripotent stem cells | Greater expression in OASC than bone marrow stem cells, similar in nasal and central OASC |
| Osteocalcin (OCN)[17] | BGLAP | Bone protein secreted by osteoblasts which inhibits bone mineralization, receptors are found in adipose tissue. | Greater expression in nasal OASC |
Several advantages exist for OASC as compared to systemic ASC. OASC have significantly higher levels of SOX2 and NANOG expression (transcription factors which indicate the ability of cells to self-renew). They have been found to have a high proliferation capacity which does not decline with patient age, unlike bone marrow stem cells, and they have been found to have increased expression of BCL2L1, an anti-apoptotic gene and decreased expression of BAX, a pro-apoptotic gene, thus suggesting a neuro-protective effect[17].
Research utilizing flow cytometry, polymerase chain reaction, and immunocytochemical staining has compared the mesenchymal stem cell marker expression in OASC derived from nasal versus central fat pads[17]. Nasal OASC were found to have higher levels of cyclin D1 compared to central OASC. When grown in normal growth media, neural gene proliferation markers Nestin and beta-tubulin III were significantly higher in nasal OASC compared to central OASC. This may be attributed to the nasal fat pad’s origins as neuroectodermal tissue rather than mesoderm. When grown in neuronal differentiation conditions, however, both nasal and medial OASC both were able to differentiate into neuroectodermal-like cells[17].
Orbital Adipose Stem Cells and Thyroid-Associated Orbitopathy
Thyroid-associated orbitopathy (TAO) is a potentially debilitating autoimmune disease characterized by simultaneous adipogenesis and fibrosis. Understanding the role of OASC may help elucidate molecular mechanisms underlying the pathophysiology of thyroid-associated orbitopathy[18].
In TAO, adipogenesis and fibrosis are regulated by separate pathways from unique fibroblast phenotypes[19]. Inflammation and edema in TAO can lead to increased intraorbital pressure, from 4 mmHg in healthy orbits to up to 27 mmHg in severe TAO[19]. While both TAO fibroblasts and control fibroblasts have been shown to differentiate into adipocytes under conditions simulating increased intraorbital pressure, TAO fibroblasts also spontaneously differentiate into adipocytes, leading to adipogenesis in TAO that may be worsened by increased intraorbital pressure[19]. TAO OASC have also been found to have downregulated WNT signaling genes. WNT signaling regulates the differentiation of neural crest progenitor cells; decreased WNT signaling in TAO OASC suggests that more OASC are committed to becoming adipocytes and can lead to hypertrophy of orbital adipose tissue[18].
TAO fibroblasts have been demonstrated to have cell surface markers that more closely resemble typical mesenchymal stem cells than control fibroblasts[20]. Studies of orbital fibroblasts reveal high expression of CD90 and absence of CD45, which represents a mesenchymal stem cell-like profile (Li), as CD90 is a hallmark of mesenchymal stem cells[20]. However, there is conflicting literature regarding the expression of CD90 in TAO orbital fibroblasts[19] [21]. TAO OASC have been found to have higher adipocyte differentiation and adipogenesis capacity, and lower osteogenic and chondrogenic capacity, when compared to healthy controls[18]. While orbital adipose tissue in TAO patients, without differentiation based on origin in nasal or central fat pad, has been studied compared to healthy controls[3] [18] [22], further research is needed to differentiate the involvement of the embryologically distinct fat pads.
Orbital Adipose Stem Cells and Corneal Wound Healing
OASC may also play a role in corneal wound healing[23] [24]. When mixed with corneal epithelial cells, OASC may differentiate from spindle-shaped, fibroblast-like cells into round, polygonal epithelial-like cells[24]. Studies in mice with alkali-induced corneal injuries show that OASCs promoted corneal re-epithelialization and reduced corneal edema and stromal infiltration. These cells can be applied topically to the cornea to facilitate corneal healing and inhibit corneal inflammation to prevent corneal haze[15]. More recently, a study of ethanol-induced corneal wound healing in a mouse model demonstrated expedited clearance of neutrophils with application of human ASC[25]. Human OASC have also been shown to improve corneal endothelial cell proliferation and repair capacity in rabbit and monkey models[26].
Conclusion
Orbital adipose tissue is a complex entity with a reservoir of stem cells. The eyelid fat pads have different embryologic origins, with the upper eyelid fat pad being derived from neuroectoderm while the central fat pad is derived from mesoderm. More accessible than bone marrow cells but with similar proliferation and differentiation capacity, these cells may provide unique opportunities for further research and treatment development. The potential role of OASC as a unique stem cell repository warrants further exploration in ophthalmology and other medical specialties.
References
- ↑ Saely, C. H., Geiger, K., & Drexel, H. (2012). Brown versus white adipose tissue: A mini-review. Gerontology, 58(1), 15-23. doi:10.1159/000321319
- ↑ Stenkula, K. G., & Erlanson-Albertsson, C. (2018). Adipose cell size: Importance in health and disease. American Journal of Physiology.Regulatory, Integrative and Comparative Physiology, 315(2), R284-R295. doi:10.1152/ajpregu.00257.2017 [doi]
- ↑ Jump up to: 3.0 3.1 Zhang, L., Evans, A., von Ruhland, C., Draman, M. S., Edkins, S., Vincent, A. E., . . . Dayan, C. M. (2020). Distinctive features of orbital adipose tissue (OAT) in graves' orbitopathy. International Journal of Molecular Sciences, 21(23), 9145. doi: 10.3390/ijms21239145. doi:10.3390/ijms21239145 [doi]
- ↑ Afanaseva, D. S., Gushchina, M. B., & Borzenok, S. A. (2018). Comparison of morphology of adipose body of the orbit and subcutaneous fat in humans. Bulletin of Experimental Biology and Medicine, 164(3), 394-396. doi:10.1007/s10517-018-3997-x [doi]
- ↑ Jump up to: 5.0 5.1 Byun, T. H., Kim, J. T., Park, H. W., & Kim, W. K. (2011). Timetable for upper eyelid development in staged human embryos and fetuses. Anatomical Record (Hoboken, N.J.: 2007), 294(5), 789-796. doi:10.1002/ar.21366 [doi]
- ↑ Kakizaki, H., Malhotra, R., & Selva, D. (2009). Upper eyelid anatomy: An update. Annals of Plastic Surgery, 63(3), 336-343. doi:10.1097/SAP.0b013e31818b42f7 [doi]
- ↑ Jump up to: 7.0 7.1 Sires, B. S., Saari, J. C., Garwin, G. G., Hurst, J. S., & van Kuijk, F. J. (2001). The color difference in orbital fat.Archives of Ophthalmology (Chicago, Ill.: 1960), 119(6), 868-871. doi:els00005 [pii]
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Cho, R. I., & Kahana, A. (2021). Embryology of the orbit. Journal of Neurological Surgery. Part B, Skull Base, 82(1), 2-6. doi:10.1055/s-0040-1722630 [doi]
- ↑ Jump up to: 9.0 9.1 9.2 Chen, S., Mahabole, M., Horesh, E., Wester, S., Goldberg, J. L., & Tseng, S. C. G. (2014). Isolation and characterization of mesenchymal progenitor cells from human orbital adipose tissue. Investigative Ophthalmology & Visual Science, 55(8), 4842-4852. doi:10.1167/iovs.14-14441 [doi]
- ↑ Massry, G. (2012). "The inverse shoe shine sign" in transconjunctival lower blepharoplasty with fat repositioning. Ophthalmic Plastic and Reconstructive Surgery, 28(3), 234-235. doi:10.1097/IOP.0b013e318248eb0f [doi]
- ↑ Jump up to: 11.0 11.1 Bronner, M. E., & LeDouarin, N. M. (2012). Development and evolution of the neural crest: An overview.Developmental Biology, 366(1), 2-9. doi:10.1016/j.ydbio.2011.12.042 [doi]
- ↑ Tawfik, H. A., & Dutton, J. J. (2018). Embryologic and fetal development of the human orbit. Ophthalmic Plastic and Reconstructive Surgery, 34(5), 405-421. doi:10.1097/IOP.0000000000001172 [doi]
- ↑ Jump up to: 13.0 13.1 Ding, D., Shyu, W., & Lin, S. (2011). Mesenchymal stem cells. Cell Transplantation, 20(1), 5-14. doi:10.3727/096368910X [doi]
- ↑ Ho, J. H., Ma, W., Tseng, T., Chen, Y., Chen, M., & Lee, O. K. (2011). Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Engineering. Part A, 17(1-2), 255-266. doi:10.1089/ten.TEA.2010.0106 [doi]
- ↑ Jump up to: 15.0 15.1 Lin, K., Loi, M., Lien, G., Cheng, C., Pao, H., Chang, Y., . . . Ho, J. H. (2013). Topical administration of orbital fat-derived stem cells promotes corneal tissue regeneration. Stem Cell Research & Therapy, 4(3), 72. doi:10.1186/scrt223 [doi]
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 16.5 Korn, B. S., Kikkawa, D. O., & Hicok, K. C. (2009). Identification and characterization of adult stem cells from human orbital adipose tissue. Ophthalmic Plastic and Reconstructive Surgery, 25(1), 27-32. doi:10.1097/IOP.0b013e3181912292 [doi]
- ↑ Jump up to: 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 17.12 17.13 17.14 Mawrie, D., Bhattacharjee, K., Sharma, A., Sharma, R., Bhattacharyya, J., Bhattacharjee, H., . . . Jaganathan, B. G. (2019). Human orbital adipose tissue-derived mesenchymal stem cells possess neuroectodermal differentiation and repair ability. Cell and Tissue Research, 378(3), 531-542. doi:10.1007/s00441-019-03072-0 [doi]
- ↑ Jump up to: 18.0 18.1 18.2 18.3 Tao, W., Ayala-Haedo, J. A., Field, M. G., Pelaez, D., & Wester, S. T. (2017). RNA-sequencing gene expression profiling of orbital adipose-derived stem cell population implicate HOX genes and WNT signaling dysregulation in the pathogenesis of thyroid-associated orbitopathy. Investigative Ophthalmology & Visual Science, 58(14), 6146-6158. doi:10.1167/iovs.17-22237 [doi]
- ↑ Jump up to: 19.0 19.1 19.2 19.3 Li, H., Fitchett, C., Kozdon, K., Jayaram, H., Rose, G. E., Bailly, M., & Ezra, D. G. (2014). Independent adipogenic and contractile properties of fibroblasts in graves' orbitopathy: An in vitro model for the evaluation of treatments. PloS One, 9(4), e95586. doi:10.1371/journal.pone.0095586 [doi]
- ↑ Jump up to: 20.0 20.1 Kozdon, K., Fitchett, C., Rose, G. E., Ezra, D. G., & Bailly, M. (2015). Mesenchymal stem cell-like properties of orbital fibroblasts in graves' orbitopathy. Investigative Ophthalmology & Visual Science, 56(10), 5743-5750. doi:10.1167/iovs.15-16580 [doi]
- ↑ Khoo, T. K., Coenen, M. J., Schiefer, A. R., Kumar, S., & Bahn, R. S. (2008). Evidence for enhanced thy-1 (CD90) expression in orbital fibroblasts of patients with graves' ophthalmopathy. Thyroid : Official Journal of the American Thyroid Association, 18(12), 1291-1296. doi:10.1089/thy.2008.0255 [doi]
- ↑ Brandau, S., Bruderek, K., Hestermann, K., Görtz, G., Horstmann, M., Mattheis, S., . . . Berchner-Pfannschmidt, U. (2015). Orbital fibroblasts from graves' orbitopathy patients share functional and immunophenotypic properties with mesenchymal Stem/Stromal cells. Investigative Ophthalmology & Visual Science, 56(11), 6549-6557. doi:10.1167/iovs.15-16610 [doi]
- ↑ Wester, S. (2014). Orbital stem cells. Current Ophthalmology Reports, (2), 107. doi:10.1007/s40135-014-0044-6
- ↑ Jump up to: 24.0 24.1 Ho, J. H., Ma, W., Tseng, T., Chen, Y., Chen, M., & Lee, O. K. (2011). Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Engineering. Part A, 17(1-2), 255-266. doi:10.1089/ten.TEA.2010.0106 [doi]
- ↑ Shang, Q., Chu, Y., Li, Y., Han, Y., Yu, D., Liu, R., . . . Shi, Y. (2020). Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea. Cell Death & Disease, 11(8), 707-020-02914-y. doi:10.1038/s41419-020-02914-y [doi]
- ↑ Sun, P., Shen, L., Zhang, C., Du, L., & Wu, X. (2017). Promoting the expansion and function of human corneal endothelial cells with an orbital adipose-derived stem cell-conditioned medium. Stem Cell Research & Therapy, 8(1), 287-017-0737-5. doi:10.1186/s13287-017-0737-5 [doi]