All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
ICD 9
- Optic nerve hypoplasia 377.43
ICD 10
- Optic nerve hypoplasia, right eye H47.031
- Optic nerve hypoplasia, left eye H47.032
- Optic nerve hypoplasia, bilateral H47.033
Disease
Optic nerve hypoplasia (ONH) is characterized by decreased number of optic nerve axons. It can present unilaterally or bilaterally.[1] It may present as an isolated anomaly or be associated with midline cerebral structural defect, such as septum pellucidum absence, agenesis of corpus callosum, cerebral hemisphere abnormalities, or pituitary gland abnormalities. A high proportion of patients with ONH have concurrent structural central nervous system abnormalities (90%) and neuro-developmental handicaps (70%).[2]
Septo–optic dysplasia (SOD, de Morsier syndrome) is used to describe the association between ONH and the absence of septum pellucidum, deficiency of pituitary hormones and agenesis of corpus callosum. Clinical manifestations of septo-optic dysplasia include visual impairment, hypopituitarism, and developmental delays. Gillespie syndrome is a genetic disease that presents as a triad of aniridia, cerebellar ataxia, and intellectual disability; it is also sometimes accompanied by other ocular findings such as ONH.[3][4]
ONH that only involves the superior segment is termed Superior Segmental Optic Nerve Hypoplasia (SSONH). ONH that only involves the nasal quadrant is termed nasal optic disc hypoplasia. Bilateral SSONH may be associated with maternal diabetes mellitus.
History
The first case of ONH was described by Briere in 1877, and the first schematic drawing of the appearance of the optic disc was done by Schwarz in 1915. In 1941, Dr. David Reeves at Children’s Hospital Los Angeles first described the association of ONH with agenesis of the septum pellucidum. And subsequently the term of “la dysplasia septo- optique” was described by Georges de Morsier in 1956. In 1970 Dr. William Hoyt’s landmark paper recognized the association between ONH and growth hormone deficiency. Dr. Hoyt attributed the discovery of the association of optic nerve hypoplasia with septum pellucidum agenesis to de Morsier, and resurrected the term septo-optic dysplasia syndrome.[7]
Epidemiology
In 2007, ONH was considered the 3rd most prevalent cause of vision impairment in children less than 3 years of age[8] following cortical vision impairment and retinopathy of prematurity. Optic nerve hypoplasia was identified in 12% of blind infants in Harris County in Texas in early 1980s.[9] In more recent studies, the incidence of ONH in all living children under the age of 18 was reported to be 10.9 per 100,000 in England and 17.3 per 100,000 in Sweden.[10][11] In 2013, a study based in Mayo Clinic reported an incidence of 1/2287 live births and 2.4/100,000 in patients less than 19 years old. ONH is usually diagnosed at 2 years of age.[12]
Genetics
A number of gene mutations have been associated with ONH:
| Gene | Function | Clinical feature/Associated disorders |
|---|---|---|
| HESX1 | Transcription regulation gene that is critical
to the development of the forebrain, midline brain structures, and pituitary |
Combined pituitary hormone deficiency or
septo-optic dysplasia[13] |
| PAX6 | Transcription regulation gene that guides neural
and ocular development |
Abnormalities
in the iris, nystagmus and foveal hypoplasia, cataracts, corneal abnormalities, glaucoma and bilateral ptosis[14] |
| SOX2 | Transcription factor that plays an important
role in embryogenesis |
Bilateral ONH, absent septum pellucidum,
bilateral schizencephaly, right porencephalic cyst[15] |
| SOX5 | Transcription factor that plays an important
role in neurogenesis |
Lamb-Schaffer syndrome (ONH, optic atrophy, developmental delay, behavioral problems, poor expressive speech, mild dysmorphic features, and skeletal abnormalities)[16]. |
| COL4A1/COL4A2 | Protein coding genes that encode the alpha-1 and alpha-2 subunits of collagen type IV, essential for basement membranes and cell adhesion, migration, proliferation, and differentiation | Small-vessel brain disease (porencephaly and schizencephaly), renal disease, muscular disease, and cerebrovascular disease. Ophthalmological disorders include retinal arterial tortuosity, congenital cataract, anterior segment dysgenesis, glaucoma, microphthalmia/anophthalmia, optic atrophy, and ONH[16]. |
| KIF7 | Protein coding gene that is an important regulator for the sonic hedgehog signaling pathway, which is responsible for patterning and brain development | Joubert syndrome, acrocallosal syndrome[16]. |
| CASK | X-linked gene involved in pre- and postsynaptic neuronal signaling[17] | Intellectual disability, microcephaly, pontocerebellar hypoplasia, ONH, and seizures[18]. |
| MED12 | Gene that encodes a significant portion of RNA polymerase II, which is critical for gene transcription | Ohdo syndrome, Lujan-Fryns syndrome, Opitz-Kaveggia syndrome, and ocular features such as ptosis, hypertelorism, strabismus, astigmatism, and ONH[19]. |
| NR2F1 | Transcription regulation gene that is critical
to neurodevelopment, optic development, oligodendrocyte differentiation, etc |
Intellectual disability seen with ONH/optic
atrophy[20] |
| OTX2 | Transcription factor that affects brain
development. Family screening for a possible mutation is important as mutations tend to be hereditary |
Anophthalmia/microphthalmia,
brain malformations, pituitary abnormalities; short stature, intellectual disabilities, feeding difficulties[21] |
| VAX1 | Transcription regulation gene in eye development | Bilateral microphthalmia, cleft
lip/palate, corpus callosum defects, hippocampal defects, absent pineal gland[22] |
| ATOH7 | Transcription regulation gene that controls
ocular development and formation of retinal ganglion cells |
Smaller optic disc[23] |
| KANSL1 | Transcription regulation through histone
acetylation |
Strabismus, astigmatism, myopia[24] |
Risk Factors
It is hypothesized that ONH is a result of vascular disruption, primarily of the proximal trunk of the anterior cerebral artery[25] and this hypothesis is supported by risk factors for ONH such as increased first trimester bleeding and maternal smoking.[2] Other risk factors include young maternal age, maternal diabetes, preterm labor, and primiparity. [26][12] Additionally, low maternal weight gain, and maternal weight loss during the first and second trimesters increase the risk of ONH. Usage of drugs during the pregnancy may also play a role. These drugs/substances include alcohol, recreational substances including LSD (lysergic acid diethylamide), protamine zinc insulin, meperidine, anticonvulsants, diuretics, corticosteroids, lithium, and antidepressants. Recent studies, however, demonstrate that maternal use of alcohol, recreational drugs, anticonvulsants, antidepressants, and viral infections during pregnancy appear to play a smaller role in the development of ONH than previously thought.[27]
General Pathology
In ONH, retinal nerve fiber layer and ganglion cell numbers may be decreased whereas the outer retinal layer is generally less affected.[28][29]
Diagnosis
Signs
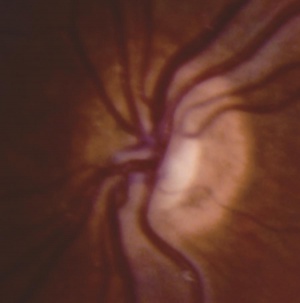
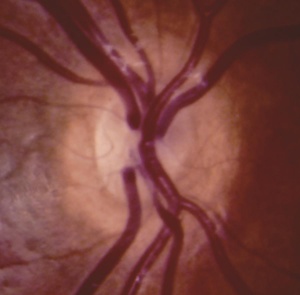
In ONH, the optic disc is often pale or gray and appears to be, at most, half the size of a normal optic disc.[30] Optic discs often present with a double ring sign – yellow to white ring around the disc. A ring of hypopigmentation or hyperpigmentation often surrounds the disc defining the area of the putative scleral canal. The outer ring represents the normal junction between the sclera and the lamina cribrosa; the inner ring represents the abnormal extension of retina and pigment epithelium over the outer portion of the lamina cribrosa. Tortuous retinal arterioles, venules, or both may accompany ONH, but retinal vessels can also present with normal caliber. The ratio of the horizontal disc diameter (DD) to the distance between the macula and the temporal edge of the disc (DM) constitutes the DD/DM ratio; DD/DM ratios less than 0.35 are generally indicative of ONH.[31] Clinically, the distance between the temporal margin of the disc is usually more than 3 times the disc diameter. However, patients’ age needs to be considered when evaluating the DD/DM ratio. For example, the normal DD/DM ratio in premature infants has been reported as ≥ 0.26.[32] Additionally, Optical Coherence Tomography (OCT) studies may reveal foveal thinning of several retinal layers including the RNFL (retinal nerve fiber layer), GCL (ganglion cell layer), IPL (inner plexiform layer), OPL (outer plexiform layer), ONL (outer nuclear layer), and IS (inner segment) layers. The retinal changes observed are similar to foveal hypoplasia; however, ONH results in more severe changes in the RNFL and GCL retinal layers.[28] Refractive errors, astigmatism, dyschromatopsia, and amblyopia may be associated. Associated retinal venous tortuosity in bilateral ONH may point toward endocrine abnormalities.
Symptoms
In ONH, visual acuity can range from normal to light perception. Most patients suffer from a visual acuity of 20/200 or worse.[30] Visual acuity in ONH patients is related to the structure of the macula and is often not correlated with overall size of the disc.[28] Visual field often has localized defects combined with visual field constrictions, commonly in nasal or inferior fields.[29] ONH can occur unilaterally, bilaterally symmetric, or bilaterally asymmetric. In unilateral and asymmetric bilateral ONH, relative afferent pupillary defects can be seen as well. Congenital sensory nystagmus often presents in bilateral ONH cases at 1-3 month of age, followed by strabismus development by 1 year of age, commonly esotropia. Strabismus often develops in markedly asymmetric or unilateral cases. Hypothalamic dysfunction is the most common nonvisual problem in ONH cases and is reported to be present in 69% of unilateral cases vs 81% of bilateral cases.[33] Absence of the septum pellucidum was not associated with poor vision, pituitary dysfunction or developmental delays.[34] It has been reported that 13-34% of optic nerve hypoplasia cases have pituitary abnormalities: empty sella, ectopic posterior pituitary, non-visualized infundibulum and posterior pituitary. Endocrine abnormalities are associated with the absence of the infundibulum which results in the lack of communication between the hypothalamus and the anterior pituitary. The hypothalamus is then unable to properly stimulate the anterior pituitary leading to low levels of anterior pituitary hormones and hypopituitarism. The following hormonal dysfunctions have been seen in patients with ONH[30]: growth hormone deficiency (70%), moderately elevated serum prolactin (normally suppressed by hypothalamus), hypothyroidism (43%); adrenocorticotropic hormone deficiency (27%) leading to hypercortisolism, and diabetes insipidus (5%) due to posterior pituitary abnormalities. Normal pituitary function at initial evaluation does not preclude development of endocrinopathy in the future. Additionally, early diagnosis of endocrine dysfunction and proper treatment with hormone replacement therapy is critical to reducing mortality. Developmental delays occur in 75% of ONH cases,[33][35][36] with higher incidence in bilateral cases (78%) vs unilateral cases (39%). Patients can present with motor delays (75%) and communication delays (44%). Other abnormalities include hypoglycemia (transient or permanent), microgenitalism, and hyperbilirubinemia.[37]
Diagnostic procedures/Laboratory test
Magnetic resonance imaging (MRI) studies are recommended in patients with suspected ONH. The diameter of the intracranial optic nerve and thinning of the optic chiasm can be assessed on MRI scans. Additionally, the visualization presence of midline abnormalities can be visualized to clinically confirm the diagnosis of SOD.[30] Optical coherence tomography (OCT) is also an effective tool for measuring foveal parameters and detecting ONH.[28] However, all cases of ONH may not be diagnosed with radiographic measurements alone.[31]
Differential diagnosis
The differential diagnoses include optic nerve atrophy, optic nerve coloboma, peripapillary staphyloma, morning glory disc anomaly, tilted disc syndrome, and glaucoma.
Management
An ophthalmic evaluation is recommended for all neonates with jaundice and recurrent hypoglycemia, as these may be early signs of hypothalamic dysfunction. Additionally, an ophthalmic evaluation is recommended for infants with poor visual behavior, strabismus, or nystagmus by 3 months of age. MRI brain is recommended for confirmed ONH cases to evaluate for hydrocephalus, corpus callosum hypoplasia, schizencephaly, or polymicrogyria. Positive MRI brain should prompt a neurologic exam. Endocrinologic and pituitary function evaluations are recommended regardless of the status of the septum pellucidum. Endocrine workups include: fasting morning cortisol and glucose, TSH (thyroid stimulating hormone), free T4 (thyroxine), growth hormone surrogates (IGF-1 or insulin-like growth factor 1, IGFBP-3 or IGF binding protein 3), LH (luteinizing hormone), FSH (follicle stimulating hormone), and testosterone (if < 6 months age).[31]
General treatment
All ONH patients with central nervous system abnormalities or endocrinopathies would benefit from a multi-disciplinary team of physicians including a neurologist, neurosurgeon, endocrinologist and/or other specialists from respective fields such as occupational, physical and speech therapists. The recommended follow-up is semi-annually for growth patterns and annually for visual function. Surgical correction for strabismus is reserved for patients with symmetrical functional vision in both eyes, and potential for binocularity. Otherwise, surgical strabismus may be deferred.[31]
References
- ↑ Kanski J, Clinical Ophthalmology, A Systemic Approach. 6th edition. 2007.
- ↑ 2.0 2.1 Tornqvist K, Ericsson A, Kallen B. Optic nerve hypoplasia: risk factors and epidemiology. Acta Ophthalmol Scand. 2002; 80: 300-304
- ↑ Nabih O, Hamdani H, ELMaaloum L, Allali B, ELkettani A. Gillespie syndrome: An atypical form and review of the literature. Ann Med Surg (Lond). 2022;74:103244. Published 2022 Jan 8. doi:10.1016/j.amsu.2022.103244
- ↑ Tripathy K, Salini B. Aniridia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; February 21, 2022.
- ↑ Image source: AAO
- ↑ 2 Image source: AAO
- ↑ Hoyt WF, Kaplan SL, Grumbach MM, Glaser JS. Septo-optic dysplasia and pituitary dwarfism. Lancet. 1970;1:893–894
- ↑ Hatton D, Schwietz E, Boyer B, Rychwalski P. Babies Count: the national registry for children with visual impairments, birth to 3 years. J AAPOS. 2007;11:351–355.
- ↑ Williamson WD, Desmond MM, Andrew LP, Hicks RN. Visually impaired infants in the 1980s. A survey of etiologic factors and additional handicapping conditions in a school population. Clin Pediatr. 1987;26:241–244.
- ↑ Patel L, McNally R, Harrison E, Lloyd IC, Clayton PE. Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J Pediatr. 2006;148:85–88
- ↑ Teär Fahnehjelm K, Dahl S, Martin L, et al. Optic nerve hypoplasia in children and adolescents; prevalence, ocular characteristics and behavioural problems. Acta Ophthalmologica. 2014;92:563-570.
- ↑ 12.0 12.1 Mohney BG, Young RC, Diehl N. Incidence and Associated Endocrine and Neurologic Abnormalities of Optic Nerve Hypoplasia. JAMA Ophthalmology. 2013;131:898-902
- ↑ Dattani MT, Martinez-Barbera JP, Thomas PQ, Brickman JM, Gupta R, Mårtensson IL, Toresson H, Fox M, Wales JK, Hindmarsh PC, Krauss S, Beddington RS, Robinson IC. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet 1998;19:125–33.
- ↑ Hingorani M., Kathleen A. Williamson, Anthony T. Moore, Veronica van Heyningen; Detailed Ophthalmologic Evaluation of 43 Individuals with PAX6 Mutations. Invest. Ophthalmol. Vis. Sci. 2009;50(6):2581-2590. doi: 10.1167/iovs.08-2827.
- ↑ Kelberman D, Rizzoti K, Avilion A, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. The Journal of clinical investigation. 2006;116:2442-2455.
- ↑ 16.0 16.1 16.2 Dahl S, Pettersson M, Eisfeldt J, et al. Whole genome sequencing unveils genetic heterogeneity in optic nerve hypoplasia. PLoS One. 2020;15(2):e0228622. Published 2020 Feb 10. doi:10.1371/journal.pone.0228622
- ↑ Becker, M., Mastropasqua, F., Reising, J.P. et al. Presynaptic dysfunction in CASK-related neurodevelopmental disorders. Transl Psychiatry 10, 312 (2020). https://doi.org/10.1038/s41398-020-00994-0
- ↑ Patel, P. A. et al. Haploinsufficiency of X-linked intellectual disability gene CASK induces post-transcriptional changes in synaptic and cellular metabolic pathways. Exp. Neurol. 329, 113319 (2020)
- ↑ Shah A, Bapna M, Al-Saif H, Li R, Couser NL. Eye and ocular adnexa manifestations of MED12-related disorders. Ophthalmic Genet. 2022;43(1):126-129. doi:10.1080/13816810.2021.1989601
- ↑ 13 Bosch DGM, Boonstra FN, Gonzaga-Jauregui C, et al. NR2F1 Mutations Cause Optic Atrophy with Intellectual Disability. American Journal of Human Genetics. 2014;94:303-309.
- ↑ 14 Schilter K, Schneider A, Bardakjian T, et al. OTX2 microphthalmia syndrome: four novel mutations and delineation of a phenotype. Clinical Genetics. 2011;79:158-168.
- ↑ Slavotinek AM, Chao R, Vacik T, et al. VAX1 mutation associated with microphthalmia, corpus callosum agenesis, and orofacial clefting: The first description of a VAX1 phenotype in humans. Human Mutation. 2012;33:364-368.
- ↑ 16 Macgregor S, Hewitt AW, Hysi PG, et al. Genome-wide association identifies ATOH7 as a major gene determining human optic disc size. Human molecular genetics. 2010;19:2716-2724.
- ↑ Zollino M, Marangi G, Ponzi E, et al. Intragenic KANSL1 mutations and chromosome 17q21.31 deletions: broadening the clinical spectrum and genotype-phenotype correlations in a large cohort of patients. Journal of medical genetics. 2015;52:804.
- ↑ Lubinsky MS. Hypothesis: septo-optic dysplasia is a vascular disruption sequence. Am J Med Genet. 1997;69(3):235-236.
- ↑ Atapattu N, Ainsworth J, Willshaw H, et al. Septo-optic dysplasia: antenatal risk factors and clinical features in a regional study. Horm Res Paediatr. 2012;78(2):81-87. doi:10.1159/000341148
- ↑ Garcia-Filion P, Fink C, Geffner ME, Borchert M. Optic nerve hypoplasia in North America: A re-appraisal of perinatal risk factors. Acta Ophthalmologica. 2010;88:527-534.
- ↑ 28.0 28.1 28.2 28.3 Pilat A, PhD, Sibley D, BMBS, McLean RJ, MSc, Proudlock FA, PhD, Gottlob I, MD. High-Resolution Imaging of the Optic Nerve and Retina in Optic Nerve Hypoplasia. Ophthalmology. 2015;122:1330-1339.
- ↑ 29.0 29.1 Ouvrier R, Billson F: Optic nerve hypoplasia: a review. Journal of child neurology 1986, 1(3):181-188.
- ↑ 30.0 30.1 30.2 30.3 Smith PM, Rismondo V. Diagnosing Septo-Optic Dysplasia. https://www.aao.org/eyenet/article/diagnosing-septo-optic-dysplasia. Accessed April 23, 2018.
- ↑ 31.0 31.1 31.2 31.3 Borchert M, Garcia-Filion P. The syndrome of optic nerve hypoplasia. Curr Neurol Neurosci Rep. 2008;8(5):395-403. doi:10.1007/s11910-008-0061-7
- ↑ Kaur S, Jain S, Sodhi HBS, Rastogi A, Kamlesh. Optic nerve hypoplasia. Oman journal of ophthalmology. 2013;6:77-82.
- ↑ 33.0 33.1 Garcia-Filion P, Epport K, Nelson M, Azen C, Geffner ME, Fink C, Borchert M. Neuroradiographic, endocrinologic, and ophthalmic correlates of adverse developmental outcomes in children with optic nerve hypoplasia: a prospective study. Pediatrics. 2008;121:e653–e659
- ↑ Ahmad T, Garcia-Filion P, Borchert M, Kaufman F, Burkett L, Geffner M. Endocrinological and auxological abnormalities in young children with optic nerve hypoplasia: a prospective study. J Pediatr. 2006 Jan;148(1):78-84.
- ↑ Margalith D, Jan JE, McCormick AQ, Tze WJ, Lapointe J. Clinical spectrum of optic nerve hypoplasia: a review of 51 patients. Dev Med Child Neurol. 1984;26:311–322
- ↑ Burke JP, O'Keefe M, Bowell R. Optic nerve hypoplasia, encephalopathy, and neurodevelopmental handicap. Br J Ophthalmol. 1991 Apr;75(4):236-9. PubMed PMID: 2021594; PubMed Central PMCID: PMC1042331.
- ↑ Ryabets-Lienhard A, Stewart C, Borchert M, Geffner ME. The Optic Nerve Hypoplasia Spectrum: Review of the Literature and Clinical Guidelines. Advances in pediatrics. 2016;63:127.

