Optic Nerve Sheath Decompression
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
First described by De Wecker in 1872, optic nerve sheath decompression (ONSD), also referred to as optic nerve sheath fenestration, is a surgical procedure used to relieve pressure from the subarachnoid space in the setting of increased intracranial pressure (ICP).[1] This relief of pressure is achieved by creating a slit or window in the dura mater of the optic nerve with subsequent egress of cerebrospinal fluid (CSF).
ONSD is most frequently performed in the setting of pseudotumor cerebri (PTC) syndrome, the most common form of which is idiopathic intracranial hypertension (IIH).[1] However, it may also be indicated in other conditions which cause a secondary intracranial hypertension such as cerebral venous thrombosis and cryptococcal meningitis. [1] Left untreated, elevated ICP can transmit along the optic nerve, which is encapsulated by a minimally elastic dural sheath, to the optic disc. This elevation in pressure can ultimately lead to axonal death at the optic disc and permanent visual loss.
Initial management of IIH includes medical therapy and weight loss unless a patient is presenting with fulminant visual loss.[2] Mild to moderate disease can be managed medically with oral acetazolamide, topiramate, or furosemide.[1][2] Surgical treatment of IIH is typically reserved for patients who are unable to tolerate or are non-adherent to medical therapy, develop progressive symptoms despite maximal medical therapy, or present with fulminant visual loss. In the setting of more severe disease with significant headache or visual compromise, IIH is treated with a CSF shunting procedure or ONSD, respectfully.[1][2] Although the exact mechanism behind the success of ONSD is unknown, it is believed to be due to a combination of CSF release from the subarachnoid space as well as scarring of the dural incision site which prevents future accumulation of CSF.[1][3]
| Surgical Procedure | CPT Code |
|---|---|
| Optic Nerve Sheath Decompression | 67570 |
Historical Perspective
Optic disc edema due to elevated ICP was first described in 1853 by Turck and Coccius, although the pathophysiology was not understood at that time.[4] In 1908 Parsons coined the term "papilledema” which he derived from Briggs’ 1676 erroneous description of the optic disc as a “papilla”.[4][5]
ONSD as a treatment for optic disc edema was first described by De Wecker in 1872 as a treatment for neuroretinitis. In two unanesthetized patients, De Wecker performed an inferolateral peritomy and blindly incised the retro-orbital sheath to relieve pressure.[6] [7] This was an uncommon procedure and in the following years, only 15 additional cases were reported until Hayreh demonstrated resolution of papilledema following an incision of the optic nerve sheath in 1964.[4][8]
In 1973, Galbraith and Sullivan developed a novel approach to ONSD using a medial transconjunctival incision with detachment and reinsertion of the medial rectus muscle.[1][9] In 1988, Tse et al. expanded on the lateral orbital approach developed by Davidson in 1969. He described removal of a portion of the lateral orbital wall en bloc with utilization of modified instruments for retraction and dissection within the orbit, and an operating microscope to better visualize the optic nerve.[10] This lateral orbitotomy technique has since been adapted without the need for bone removal.[1] In 2001, Pelton and Patel described a novel approach using a superomedial lid crease incision with dissection to the medial intraconal space.[11] Presently, the medial transconjunctival approach is most popular technique for ONSD but approach varies by surgeons.[7]
Anatomy
Optic Nerve
The optic nerve courses intracranially from the medial aspect of the posterior globe and is categorized anatomically into 4 sections: intraocular (1 to 2mm in length), intraorbital (24-30 mm), intracanalicular (5-10 mm), and intracranial (9-10mm). The optic nerves merge to form the optic chiasm before dividing into the optic tracts, which carry action potentials to the lateral geniculate nuclei. At this point, they branch into the optic radiations, consisting of a superior limb traveling through the parietal lobe and an inferior limb (“Meyer’s Loop”) traveling through the temporal lobe, to the occipital lobe. This pathway allows for transmission and subsequent processing of visual sensory input.[12]
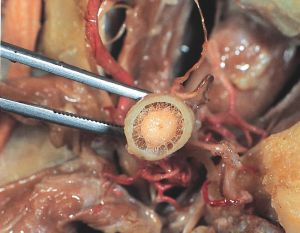
Compared to other cranial nerves, the optic nerve is unique in its development as an extension of the central nervous system (CNS) white matter. It thus mirrors the CNS’ encasement in 3 membranous meningeal layers: the pia mater, the arachnoid mater, and the dura mater. CSF flows between the pia mater directly overlying the optic nerve and the arachnoid mater in the subarachnoid space.[1][12][13] See Image 1 for a transected view of the optic nerve demonstrating the meningeal layers with the subarachnoid space.
The neuronal subarachnoid space consists of a complex arrangement of arachnoid trabeculae and septa which divide the subarachnoid space into compartments. These trabeculae and septa appear to be more densely concentrated in the immediate retro-orbital optic nerve and less dense towards the canalicular portion of the optic nerve. This architecture is postulated to play a role in the success of ONSD.[1][13] The inelastic and fibrous dura mater layer is superficial to the arachnoid mater, completing the optic nerve sheath.
Cerebrospinal Fluid
CSF is a colorless fluid that, under standard conditions, is composed of glucose (60-80% plasma glucose level), protein (20 to 40mg/100mL), and few inflammatory cells (less than 5 white blood cells). It has a total volume of approximately 150mL with nearly 500mL of CSF secreted every 24 hours. The average adult lumbar puncture opening pressure is 10 to 20 cm H2O when lying down and 20 to 30 cm H2O in a seated position. Children and infants typically have lower opening pressures than adults.[14]
CSF is primarily secreted by the epithelial cells of the choroid plexus of the lateral ventricles and the tela choroidea of the third and fourth ventricles.[14] This is accomplished by a process that transports sodium, chloride and bicarbonate from the blood to the ventricles resulting in an osmotic gradient that drives the net secretion of H2O.[15] CSF travels through the ventricular system before entering the subarachnoid space between the firmly adherent pia mater and the loosely adherent arachnoid mater.[14][15] Absorption into the venous outflow system has long been considered to occur at the cranial and spinal arachnoid villi. Experimental data is now suggesting that the cranial and spinal nerve sheaths, the cribriform plate, and the cerebral arteries’ adventitia constitute a significant outflow pathway into the lymphatic drainage system.[16]
The subarachnoid space of the optic nerve is continuous with that of the CNS, and thus elevations of ICP present in the CSF can be directly transmitted along the length of the optic nerve to the optic nerve disc. Increased intra-neuronal pressure results in defective axonal transport and subsequent intra-axonal swelling. This swelling compromises optic nerve perfusion, resulting in optic disc and nerve ischemia with loss of vision.[1][17] Visual loss often presents initially as enlargement of the physiological blind spot followed by progressive peripheral field loss.[17]
Surgical Indications
Many conditions can cause increased intracranial pressure, including pseudotumor cerebri, cerebral venous sinus thrombosis, and intracranial lesions that impair CSF outflow or absorption by the arachnoid villi.[1][13] The initial goal of treatment for elevated intracranial pressure is aimed at treatment of the underlying pathology.
The most common indication for ONSD is IIH. Conservative measures remain first line therapy and include weight loss, lifestyle modification, discontinuation of offending agents (examples include Vitamin A, tetracyclines, growth hormones), and medical management with oral Acetazolamide, Topiramate, or Furosemide.[1][2] Oral and IV steroids have been used as treatment for IIH in the past, but due to long term side effects, are largely reserved for patients with rapidly progressive vision loss while surgical plans are being made.[2]
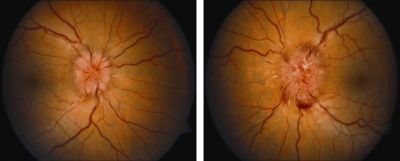
Surgical treatment of IIH is typically reserved for patients who are unable to tolerate or are non-adherent to medical therapy, develop progressive symptoms despite maximal medical therapy, or present with fulminant visual loss. Surgical methods described include ONSD, CSF diversion through lumboperitoneal (LPS) or ventriculoperitoneal shunt (VPS), venous sinus stenting (VSS), or bariatric surgery. ONSD is recommended in the setting of elevated ICP with significant papilledema and impending or progressive visual loss with minimal headaches or other neurological sequelae. See Image 2 for a demonstration of visually threatening papilledema in IIH. Patients with headache symptoms generally undergo a CSF diversion procedure.[1][2][18]
VSS has been recently developed for treatment of IIH with encouraging visual and neurological outcomes; however, more randomized clinical trials are needed to better elucidate its role in the treatment of IIH. Bariatric surgery is another surgical consideration for patients in whom obesity is believed to be a major contributor to disease due to its high success rate, even in patients previously refractory to both conventional medical and surgical intervention. However, it is not recommended for a fulminant presentation with concern for acute visual decompensation.[18][19]
Contraindications
ONSD is generally not indicated for patients with elevated ICP without concern for visual decline.[1][2] Medical therapy and lifestyle adjustment remain first line treatment for these patients.[1][2] Additionally, ONSD is not recommended in cases of elevated ICP with headache in the absence of visual findings, as CSF shunting procedures present better treatment options in this setting.[1][2][18] The exception to this occurs when CSF shunting is not readily available and urgent ONSD is needed to preserve vision in the immediate setting. ONSD should not be performed in patients on chronic anticoagulation due to risk of orbital hemorrhage with subsequent orbital compartment syndrome.[1] It is also contraindicated in patients with active CNS infections to prevent orbital seeding.[1]
Surgical Techniques
Although several different techniques for ONSD have been performed, the three most common techniques are described here. These three surgical approaches include a medial orbitotomy/transconjunctival approach (59% of surgical cases), a superomedial lid crease incision (31%), and a lateral orbitotomy (10%) approach.[1][7] The choice of approach is surgeon dependent. The goal of all ONSD techniques is to open a slit or window in the optic nerve sheath to allow adequate egress of CSF to reduce pressure on the nerve axons and preserve optic nerve function. The advantages and disadvantages of each technique are summarized in Table 1.
Table 1: Comparison of Optic Nerve Sheath Decompression Approach Advantages and Disadvantages
| Surgical Approach | Advantages | Disadvantages |
|---|---|---|
| Medial Transconjunctival |
|
|
| Superomedial Lid Crease |
|
|
| Lateral Orbitotomy |
|
|
For all ONSD approaches, the patient is placed under general anesthesia and prepared and draped in the usual sterile fashion for ophthalmic plastic surgery.
Technique 1: Medial Orbitotomy with Transconjunctival Approach
Described by Galbraith and Sullivan in 1973, the medial transconjunctival approach has become the most commonly performed technique for ONSD. The primary advantage of this technique is the short course from external incision to the optic nerve without a skin incision. However, this approach requires dis-insertion and re-attachment of the medial rectus which can result in restrictive or paralytic strabismus.[1][7][9]
Procedure Steps
1. After induction of general anesthesia, maximal exposure of the eye is obtained by placing an eyelid speculum.
2. A sectoral medial conjunctival peritomy is performed and dissection is carried down to bare sclera to expose the medial rectus muscle insertion.
3. The medial rectus muscle is isolated with a series of muscle hooks and freed from its’ attachments to Tenon’s capsule and the overlying conjunctiva. The muscle is then imbricated with a double armed 6-0 Vicryl suture using locking bites and detached from the globe at its’ insertion.
4. A 5-0 traction suture is then passed through the stump of the medial rectus insertion, and the globe is rotated into abduction to maximize the optic nerve exposure.
5. At this time the surgeon switches to an operating microscope
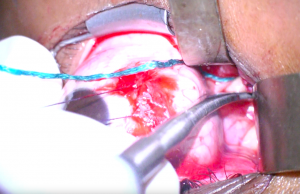
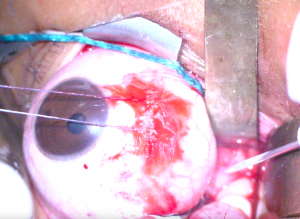
6. Blunt dissection is carried out posteriorly along the medial side of the globe taking care to protect the medial rectus with a malleable retractor (Figure 1). Moist cottonoids are helpful for retracting fat entering the operative field of view.
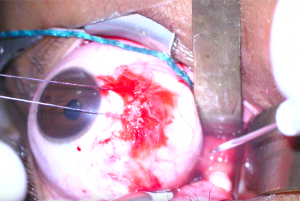
7. The long ciliary vessels are identified and followed posteriorly towards the optic nerve which will appear white compared to orbital fat. Care should be taken to avoid incising this vasculature. Malleable retractors are used to maximize exposure and visualization of the optic nerve (Figure 2).
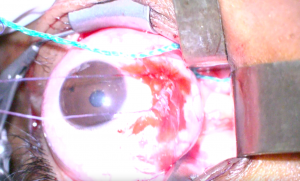
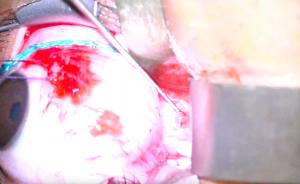
8. An incision is then made in the optic nerve sheath at least 1mm from its insertion to the posterior globe with a sharp blade or microscissors (Figure 3). A wave of CSF indicates successful incision (Figure 4).
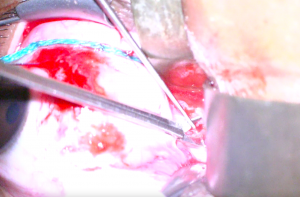
10. A blunt nerve hook or Adson hook is inserted into the incision, and the dura is tented away from the underlying optic nerve (Figure 5). A portion of the tented dura is excised with long Vannas or Grieshaber scissors (Figure 6). The amount of dura excised varies among surgeons.
11. Meticulous hemostasis is obtained with the bipolar cautery and additional hemostatic agents as needed.
12. The traction suture is removed, and the medial rectus is then reattached to its’ insertion with the double armed 6-0 Vicryl suture. The overlying conjunctiva is then closed with 6-0 or 7-0 plain gut or Vicryl suture in an interrupted fashion.
Technique 2: Superomedial Lid Crease Incision Approach
Described by Pelton and Patel in 2001, this technique utilizes an eyelid crease incision to access the medial intraconal space and the retro-orbital optic nerve. This approach allows for a shorter surgery, minimal risk of strabismus, and a good cosmetic outcome. However, this approach involves an increased distance from the incision site to the optic nerve and can therefore be more technically challenging to perform.[1][11]
Procedure Steps
1. The upper eyelid crease is marked prior to injection of local anesthetic. Following induction of general anesthesia, local anesthetic with epinephrine is injected into the incision site.
2. An incision is then made in the medial one-half to two-thirds of the upper eyelid crease. A combination of sharp and blunt dissection is used to raise a myocutanaeous flap and the orbital septum is opened. Hemostasis can be achieved with bipolar cautery.
3. Skin hooks can be used to keep the wound open. The medial horn of the levator muscle is pushed laterally. Once orbital fat is encountered, malleable retractors and blunt dissection with scissors and cotton-tipped applicators are used to gently spread the pre aponeurotic and medial fat pad while dissecting in a lateral and inferior direction toward the posterior aspect of the globe.
4. The optic nerve is identified in the superomedial quadrant of the globe and cotton-tipped applicators are used to gently push the posterior ciliary vessels off the nerve. Care should also be taken to avoid injury to the superior ophthalmic and vortex veins.
5. Using standard fenestration instruments, an incision is then made in the optic nerve sheath and a window may be created as previously described above.
6. Following creation of the optic nerve sheath window, hemostasis is obtained and instruments are removed.
7. The skin incision is closed using 6-0 plain gut suture in an interrupted fashion.
Technique 3: Lateral Orbitotomy Approach
The lateral orbitotomy approach to ONSD was initially described by De Wecker in 1872 and adapted by Tse et al in 1988. It has since been adapted so that removal of the lateral orbital wall is no longer necessary. While this approach provides excellent visualization of the optic nerve, the surgical procedure can be more time-consuming. This procedure also carries an increased risk of injury to the ciliary ganglion due to its’ anatomic location between the optic nerve and lateral rectus muscle.[1][10][20]
Procedure Steps
1. After induction of general anesthesia, a lateral canthotomy and inferior cantholysis is performed.
2. Traction sutures may be placed through the margin of the upper and lower eyelids to maximize exposure. An additional traction suture may be placed through the insertion of the lateral rectus muscle to facilitate adduction of the globe.
3. The temporal conjunctiva is then incised using Wescott scissors between the lateral rectus muscle and the lacrimal gland.
4. Blunt dissection is then directed posteriorly around the globe until intramuscular septa of the cone is reached, which must then be incised.
5. At this time, an operating microscope or surgical loupes are used to visualize the optic nerve. Blunt dissection is continued through the intraconal fat until the optic nerve is identified. Care should be taken to avoid injury to the ciliary ganglion, situated on the lateral aspect of the posterior optic nerve.
6. An incision and/or window is made in the optic nerve sheath as described above.
7. Hemostasis is obtained and the lateral canthotomy wound is repaired.
Post-Operative Course
Optic nerve sheath decompression is generally performed in an outpatient setting under general anesthesia. As such, recovery times tend to be relatively short. Antibiotic ointment is applied to the incision site post operatively and 2-3 times daily for at least one week.
Outcomes
ONSD is indicated for patients with visual compromise from papilledema in the setting of elevated ICP without headaches or other neurological sequelae.[1][18] The goal is to prevent further decompensation of visual acuity and restore lost visual acuity or visual field.[1] Most studies evaluating the outcomes of ONSD are in the setting of IIH management.
In 2017, Kalyvas et al conducted a meta-analysis of 15 case series and 3 case reports constituting 341 patients and 525 ONSD procedures for the treatment of IIH. They identified that ONSD was successful in improving visual fields in 64% of patients and visual acuity in 67% of patients.[21] However, only 41% of patients reported improvement in headache symptoms .[21] The mechanism for improvement in headache from ONSD still remains to be elucidated.[1] Papilledema was noted to improve in 95% of patients, though additional studies have shown that the procedure does not lead to a significant decrease in overall ICP.[21][22] An updated meta-analysis by Kalyvas et al in 2021 consisting of 818 patients and 1365 eyes further supported these conclusions.[18] These results are in concordance with prior studies which found that ONSD may improve visual acuity in up to 94% of patients and stabilize visual fields in up to 88% of patients.[23] ONSD has also demonstrated clinical utility in preservation of vision in patients who experience progressive visual loss despite a patent and functional shunt.[24]
Traditionally, surgical creation of a window in the optic nerve sheath has demonstrated favorable outcomes; however, a modified approach to ONSD wherein several slits are cut into the nerve sheath has also been studied. Patients undergoing this modified approach generally have good outcomes with 91.3% of patients demonstrating improved visual function.[25] In cases where the slits fail to sufficiently relieve the optic nerve edema, a subsequent surgery with window excision has demonstrated success.[25]
In their large 2021 meta-analysis, Kalyvas et al reported a failure rate of approximately 9.4%, which may necessitate repeat surgery.[18] This has improved from earlier studies which estimated failure rate closer to 32%.[26] While ONSD may prevent further deterioration of visual acuity, it can fail to control symptoms in up to 17% of patients necessitating further surgery with either CSF shunting or VSS.[18] Interestingly, unilateral ONSD has been shown to be effective in reducing papilledema in both the ipsilateral and the contralateral eye.[25] This phenomenon was originally described in Rhesus monkeys in 1964 by Hayreh and again in 2011 when Alsuhaibani et al. investigated its’ effects in 62 patients with papilledema.[4][27] ONSD appears to have similar outcomes in pediatric patients compared to their adult counterparts but larger studies are needed for confirmation.[28]
Complications
Several studies have reported post-operative complications in approximately 20-45% of patients who undergo ONSD, although most were transient and benign.[18][23][29] Kalyvas et all found that while VSS appears be the surgical procedure with the lowest risk of overall complications (9.4%), ONSD has the lowest risk of severe complications (2.2%). In comparison, CSF shunting procedures appear to carry a 61.7% risk of complication overall and a 9.4% risk of severe complication.[18]
Motility disorders with binocular diplopia are the most commonly identified complication from ONSD. While previous literature reported this complication rate to be as high as 29%, Kalyvas et al found that this was closer to 8.7% in their 2021 metanalysis.[18][29] Pupillary dysfunction and anisocoria (7-11%) can occur due optic nerve injury or damage to the ciliary ganglion, the latter of which is more common when a lateral orbitotomy approach is used.[18][29] Repeat ONSD after an initial attempt’s failure is believed to have a lower chance of improving visual function and a higher risk of vascular compromise.[18][29] Kalyvas et al identified a 2.2% risk of serious adverse events, including vision loss, optic neuropathy, and cranial nerve palsies requiring strabismus surgery.[18] ONSD has been associated with a 1-2.6% risk of patients experiencing severe and permanent loss of vision, believed to be predominantly from vascular compromise such as central or branch retinal vein occlusion and injury to the posterior ciliary arteries.[23][29][30] A summary of ONSD complications and their relative frequencies in comparison to other surgical treatment of IIH is found in Table 2 adapted from Kalyvas et al (2021).[18]
Table 2: Adverse Events and Their Estimated Frequencies for Surgical Interventions of Elevated Intracranial Pressure*
| Surgery | Very Common (>10%) | Common (1-10%) | Uncommon (>0.1-1%) | Rare (≤0.1%) | Overall Risk of Adverse Events | Risk of Serious Adverse Events |
|---|---|---|---|---|---|---|
| ONSD |
|
|
|
22.2% | 2.2% | |
| CSF Shunting |
|
|
|
61.7% | 9.4% | |
| VSS |
|
|
|
9.4% | 2.3% | |
| Bariatric Surgery |
|
|
58.8% | 29.4% |
*Table Adapted From Kalyvas et al[18]
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 Adesina O, Patel BC. Optic Nerve Decompression. [Updated 2021 May 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538300/
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 2012; 83: 488-494
- ↑ Davidson SI. A surgical approach to plerocephalic disc oedema. Trans Ophthalmol Soc UK. 1970;89:669-90
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Progress in Retinal and Eye Research. 2016 Jan;50:108-144
- ↑ Parsons JH. The Pathology of the Eye, vol. IV, Hodder & Stoughton, London (1908), pp. 1349-1365.
- ↑ DeWecker L. On incision of the optic nerve in cases of neuroretintis. Rep Int Opthalmol Congr. 1872;4:11-4
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Sobel RK, Syed NA, Carter KD, Allen RC. Optic Nerve Sheath Fenestrations: Current Preferences in Surgical Approach and Biopsy. Ophthalmic Plast Reconstr Surg. 2015 Jul-Aug;31(4):310-2
- ↑ Spitze A, Lam P, Al-Zubidi N, Yalamanchili S, Lee AG. Optic nerve sheath fenestration versus shunt placement for the treatment of idiopathic intracranial hypertension. Indian Journal of Ophthalmology. Oct 2014;62(10):1015-1021
- ↑ Jump up to: 9.0 9.1 Galbraith JE, Sullivan JH. Decompression of the perioptic meninges for the relief of papilledema. Am J Ophthalmol. 1973 Nov;76(5):687-92.
- ↑ Jump up to: 10.0 10.1 Tse DT, Nerad JA, Anderson RL, Corbett JJ. Optic Nerve Sheath Fenestration in Pseudotumor Cerebri: A Lateral Orbitotomy Approach. Arch Ophthalmol. 1988;106(10):1458-1462
- ↑ Jump up to: 11.0 11.1 Pelton RW, Patel BC. Superomedial lid crease approach to the medial intraconal space: a new technique for access to the optic nerve and central space. Ophthalmic Plast Reconstr Surg. 2001 Jul;17(4):241-53.
- ↑ Jump up to: 12.0 12.1 Selhorst JB and Chen Y. The Optic Nerve. Semin Neurol 2009; 29(1): 029-035.
- ↑ Jump up to: 13.0 13.1 13.2 Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. BR J Ophthmol 2003 Jun;87(6): 777-781.
- ↑ Jump up to: 14.0 14.1 14.2 Filis AK, Aghayev K, Vrionis FD. Cerebrospinal Fluid and Hydrocephalus: Physiology, Diagnosis, and Treatment. Cancer Control. 2017 Jan;24(1):6-8
- ↑ Jump up to: 15.0 15.1 Brown PD, Davies SL, Speake T, Millar ID. Molecular Mechanisms of Cerebrospinal Fluid Production. Neuroscience. 2004;129(4):957-970
- ↑ Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. European Annals of Otorhinolaryngology, Head and Neck Diseases. 2011 Dec;128(6):309-316
- ↑ Jump up to: 17.0 17.1 Wall M. Idiopathic Intracranial Hypertension. Neurol Clin 2010 Aug; 28(3): 593-617.
- ↑ Jump up to: 18.00 18.01 18.02 18.03 18.04 18.05 18.06 18.07 18.08 18.09 18.10 18.11 18.12 18.13 18.14 Kalyvas A, Neromyliotis E, Koutsarnakis C et al. A systemic review of surgical treatments of idiopathic intracranial hypertension (IIH). Neurosurg Rev. 2021 Apr;44(2): 773-792.
- ↑ Handley JD, Baruah BP, Williams DM, et al. Bariatric surgery as a treatment for idiopathic intracranial hypertension: a systemic review. Surgery for Obesity and Related Diseases. 2015 Nov;11(6):1396-1403.
- ↑ Bhardwaj N, Joshi A. Neuroanatomy, Ciliary Ganglion [Updated 2021 Jul 26]. In: StatPearls [Internet]. Treasure Island (FL) : StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553182/
- ↑ Jump up to: 21.0 21.1 21.2 Kalyvas AV, Hughes M, Koutsarnakis C, Morris D, Kiakos F, Sakas DE, Strangalis G, Fouyas I. Efficacy, complications, and cost of surgical interventions for idiopathic intracranial hypertension: a systemic review of the literature. Acta Neurochir (Wien). 2017 Jan;159(1):33-49
- ↑ Chen, H., Zhang, Q., Tan, S., Fu, H., Farris, B., & Yang, Z. (2017). Update on the application of optic nerve sheath fenestration. Restorative Neurology And Neuroscience, 35(3), 275-286. doi: 10.3233/rnn-160693
- ↑ Jump up to: 23.0 23.1 23.2 Banta JT, Farris BK. Pseudotumor Cerebri and Optic Nerve Sheath Decompression. Ophthalmology. 2000;107(10):1907
- ↑ Kelmen SE, Sergott RC, Cioffi GA, Savino PJ, Bosley TM, Elman MJ. Modified Optic Nerve Decompression in Patients With Functioning Lumboperitoneal Shunts and Progressive Visual Loss. Ophthalmology. 1991;98(9):1449
- ↑ Jump up to: 25.0 25.1 25.2 Sergott RC, Savino PJ, Bosley TM. Modified Optic Nerve Sheath Decompression Provides Long-Term Visual Improvement for Pseudotumor Cerebri. Arch Ophthalmol. 1988;106(10):1384
- ↑ Spoor C, McHenry JG. Long-Term Effectiveness of Optic Nerve Sheath Decompression for Pseduotumor Cerebri. Arch Ophthalmol. 1993;111(5):632
- ↑ Alsuhaibani AH, Carter KD, Nerad JA, Lee AG. Effect of Optic Nerve Sheath Fenestration on Papilledema of the Operated and the Contralateral Nonoperated Eyes in Idiopathic Intracranial Hypertension. Ophthalmology. 2011 Feb;188(2):412-414.
- ↑ Thuente DD, Buckley EG. Pediatric Optic Nerve Sheath Decompression. Ophthalmology. 2005;112(4):724
- ↑ Jump up to: 29.0 29.1 29.2 29.3 29.4 Plotnik JL, Kosmorski GS. Operative Complications of Optic Nerve Sheath Decompression. Ophthalmology. 1993;100(5):683.
- ↑ Mudumbai RC. Optic nerve sheath fenestration: indications, techniques, mechanisms and, results. Int Ophthalmol Clin. 2014 Winter;54(1):43-9