Ocular Neuropathic Pain
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Definition
Ocular neuropathic pain, also referred to as corneal neuropathic pain, is a condition where corneal pain is seen in response to normally non-painful stimuli. This results from repeated direct damage to corneal nerves. Aberrant regeneration with upregulation of nociceptors responsible for processing of painful stimuli leads to hyper-responsivity and increased perception of pain in response to even normally unpainful stimuli. The distorted neuronal excitability which persists even after the tissue has healed is the basis of symptoms of self-sustained chronic corneal pain persisting even in the absence of stimuli and clinical signs – the so called corneal “pain without stain” or “phantom cornea’.[1][2][3] This condition is the ocular analogue of systemic neuropathic pain, complex regional pain syndrome or reflex sympathetic dystrophy (RSD). Ocular or corneal neuropathic pain, corneal neuropathy, corneal neuralgia, kertaoneuralgia, and corneal allodynia are all terms that are used to describe the same disease entity.
Clinical Impact
Corneal neuropathic pain negatively impacts the quality of life of patients. The unpleasant and often severe sensation of pain, light sensitivity, irritation results in impaired functioning and inability to perform activities of daily living; including reading, driving and working. The impact on physical and social functioning can be debilitating. This condition is being increasingly recognized in patients with chronic dry eye syndrome who don’t seem to respond to conventional dry eye treatment and whose severity of symptoms seem disproportionate to clinical examination. It is a particularly challenging condition in post-refractive surgery patients who suffer from chronic intractable pain. Corneal pain is also considered as a part of the complex pain syndrome and can be associated with systemic pain syndromes.[4][5][6] Associated symptoms of anxiety and depression also dramatically affect quality of life and in extreme cases can even lead to induction of suicidal thoughts.[7][8][9][10][11] Due to relatively recent recognition of the problem, poorly understood underlying mechanisms and no signs on slit lamp examination, these symptoms of pain are often unfortunately ignored by ophthalmologists.
Causes of Corneal Neuropathic Pain
Triggers that lead to corneal nerve damage result in corneal neuropathy. Damage to nerves during refractive surgeries, ocular surface diseases such as chronic dry eye disease, recurrent corneal erosions, corneal neuropathic infections such as herpetic simplex and zoster, systemic neuropathic conditions such as diabetes, exposure to topical and systemic drugs, radiation keratopathy and chemotherapy.[11][12]Figure 1.
Pathophysiology
Corneal Neurobiology
Anatomy of Corneal Nerves
The human cornea is one of the most densely innervated tissues. The density of central corneal nerve endings is estimated to be around 7000 nerve terminals per square millimeter. The corneal density of nociceptors in nerve endings is about 300-600 times that of skin and 20-40 times that of tooth pulp. The corneal peripheral sensory nerves are derived from the ophthalmic division of the trigeminal nerves. These nerve endings are initially myelinated. They penetrate the corneal epithelium and Bowman’s layer in a radial fashion 1mm from the corneo – scleral limbus and lose myelination at the site of penetration. After penetration, majority of the nerves turn 90 degrees and run parallel to the epithelial surface in the subbasal layer forming the corneal subbasal corneal nerve plexus. These subbasal nerves run towards the corneal center in a clockwise centripetal pattern. Some of the nerves terminate as free nerve endings.[13]
Physiology of Corneal Nerves
The subbasal nerve plexus contains both - unmyelinated (80%; C fibers) and myelinated nerve fibers (20%; A-d fibers). The bulbous nerve endings contain the nociceptors. The nociceptors are functionally heterogenous and are classified based on the type of the stimuli they respond to. Polymodal nociceptors (70%, majorly C unmyelinated fibers) respond to a large variety of stimuli including heat, mechanical, endogenous and exogenous inflammatory stimuli. The other nociceptors are mechanoreceptors and cold receptors. This nociceptive system responds to various stimuli at the ocular surface and project to the central nervous system, and helps to sustain ocular surface health.[13][14][15]
Corneal Pain Pathway
Acute nociceptive pain pathway
Initially, in the presence of a noxious stimulus, the nociceptors get activated. Transduction mechanism at the nociceptors coverts the external, often mechanical stimulus into electrical impulses along the trigeminal nerve endings. These impulses are then transmitted along the corneal pain pathway - i.e. the trigeminal brainstem complex, trigeminal subnucleus interpolaris/caudalis transition region, upper cervical cord junction and posterior thalamus. These inputs are then processed in the corneal pain region of the cerebral cortex. This perception of acute pain is a protective mechanism.[16][17][18] However with repeated injury this pain pathway becomes maladaptive and loses the protective mechanism and develops into non –protective neuropathic pain. The processes involved are peripheral and central sensitization.
Peripheral Sensitization
Peripheral sensitization is the adaptation of the peripheral pain system to repeated injurious stimuli. Persistent damage and inflammation results in transition from acute nociceptive pain to chronic neuropathic pain.[19][20] Severe (often surgical – such as keratorefractive surgery) or repeated injury induces tissue inflammatory response. Pro-inflammaory mediators such as prostaglandins, cytokines and neuropeptides are released. These neuropeptides induce nerve regeneration. Unregulated and ineffective nerve sprouting at the ends of the injured nerve stumps leads to formation of traumatic neuromas. Formation of neuromas further causes sustained inflammation leading to a vicious cycle. Upregulation of ion channels, modification of intrinsic membrane potentials and lowering of the firing threshold makes the nociceptors abnormally hypersensitive to mechanical and chemical stimuli. This hypersensitivity results in aberrant firing thus generating ectopic nerve stimuli. The unregulated generation of ectopic stimuli even in the presence of sub-threshold stimuli is known as peripheral sensitization.[21]
Central Sensitization
In addition to the modulation at the level of nociceptors in the presynaptic trigeminal nerve endings, persistent inflammation and nerve damage also act post synaptically in the spinal cord causing direct depolarization along the central pain pathway.[22][23][24] These changes occurring in the CNS in response to repeated nerve stimulation is known as central sensitization. Upregulation of excitatory neurotransmitters, mainly NMDA and inhibition of inhibitory neurotransmitters is also seen. This causes more rapid response to similar stimulus in the future – known as neuronal plasticity.[25] This amplification of the neuronal hypersensitivity becomes permanent and persists despite removal of the initial stimulus and apparent healing of the injured tissue resulting in neuropathic pain. This perpetuates pain and dysesthesia even in response to stimuli which are normally non-painful. This is responsible for the “pain without stain” or phantom cornea.[2][3][11]
Clinical Features
Symptoms
Patients generally present with corneal pain of varying severity. Other symptoms are the dry eye like symptoms – foreign body sensation, burning and reflex tearing.[26] Also severe photosensitivity – photoallodynia, allodynia and dysesthesias can be seen. Focal facial dystonia and blepharospasms may also be noted. Corneal neuropathy has been shown to be associated with other chronic pain syndromes as well and patients often complain of non-ocular pain. Headaches often migraine type are reported. Additionally, these patients frequently have symptoms of anxiety, depression and apathy.[1][4][5][6][8][10][23][27][28]
Signs
No clinical findings are generally seen on slit lamp examination. The ocular surface appears healthy. However neuropathic pain may be a component of dry eye syndrome. In such cases surface staining, abnormalities on osmolarity testing and other signs of dry eye and surface disease may be present.[9]
Diagnosis
Identification of cause
Identification of the inciting insult is important. History of refractive surgery, ocular surface diseases, infections, systemic disorders, radiation exposure and topical and systemic drugs must be elicited.
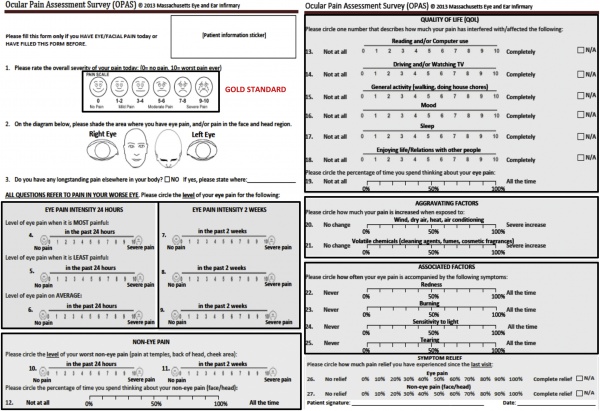
Quantification of pain
Ocular Pain Assessment Survey (OPAS) is used to quantify pain and impact on quality of life. Other dry eye questionnaires like OSDI may be used, but are not specific for pain.[29]Figure 2.
Assessment of ocular surface health and function
Ocular surface health is assessed by slit lamp examination, use of vital stains, Schirmer’s test, tear break-up time, tear osmolarity. Function of corneal nerves can be assessed using corneal esthesiometry. Increased sensitivity may support the diagnosis. Contact (Cochet-Bonnet) or non-contact (Belmonte or Brill) esthesiometry can be used.[9]
Differentiating between peripheral and central neuropathic pain
Proparacaine eye drop administration helps to differentiate whether neuropathy is peripheral only with sensitization at the level of corneal nociceptors or has progressed to central sensitization. Complete relief of pain in response to proparacaine represents purely peripheral pain however partial relief or no relief at all indicated progression to central sensitization.[9] Incomplete response to surface lubrication also indicates central component of pain.[30]
In vivo confocal microscopy
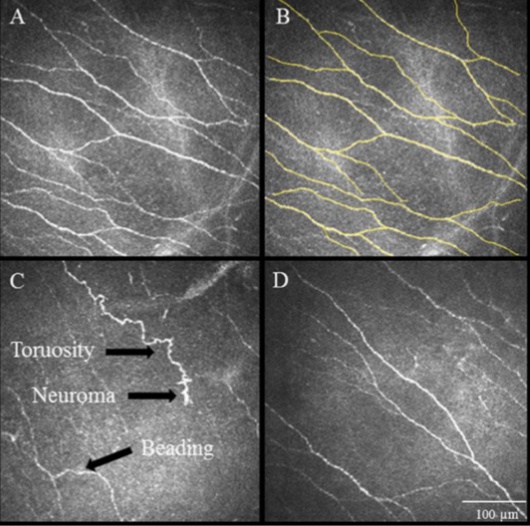
Credit: Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Aggarwal S.
Ocul Surf. 2015
In vivo confocal microscopy is a sophisticated technique which enables in vivo, ultra-high resolution quasi-histological evaluation of the corneal ultrastructure. It is a very useful for diagnosis of corneal neuropathy as it provides direct evidence of nerve injury and allows both qualitative and quantitative measurement.[31][32] Increased hyper-reflectivity, tortuosity, formation of beading and neuromas are the common findings observed at the subbasal nerve plexus layer.[7] (Figure 3)
Treatment
Principles and considerations in management of neuropathic pain
- Most important consideration is individualized management of neuropathic pain. Every patient needs to be graded according to etiology of corneal neuropathy and severity of symptoms. Focus on restoring the ocular surface health – by preventing further insults to the corneal nerves and decreasing ocular surface inflammation is an important starting point.
- Identification of the stage of the disease process, i.e. whether neuronal maladaptation and central sensitization has started. Neuro-regenerative strategies help in reversing damage to corneal nerve plexus. Local ocular surface treatment is generally insufficient for patients with central sensitization. More invasive central pain pathway modulation is needed if central sensitization has occurred.[33]
- Generally a multimodal therapy is appropriate with a focus of both ocular surface and nociceptor modulation. Step wise therapy with escalation depending on treatment response is used. Multi-specialty approach is needed as ocular neuropathic pain has been shown to be a part of complex pain syndromes and frequently requires inputs from pain specialists, neurologists and psychiatrists.
Management Options
Ocular surface treatment
- Eye lubrication and treatment of dry eye – like symptoms: Artificial tears are used to prevent surface dryness and provide symptomatic relief. By decreasing the hyper-osmolarity of tears, over-stimulation of the nociceptors is stopped. Tears, ointments and gels are all used depending on patient tolerance. If used more than 4 times, preservative free tears are recommended. Punctal plugs to increase the tear lake are also useful.
- Topical and systemic antibiotics to treat blepharitis are often used. Systemic fish oil or flax seed oil to treat evaporative component of dry eye disease has also been used.
- Scleral lenses: Scleral lenses, specifically prosthetic replacement of the ocular surface ecosystem (PROSE) has been shown to be effective in treatment of post LASIK corneal neuralgia. The PROSE devices are made of rigid gas permeable lenses, are custom designed and provide fluid ventilation to the cornea.[34]
Anti-inflammatory therapies
- Steroids: Short courses of topical steroids have been shown to be useful to decrease surface inflammation. This works both to stop the vicious cycle of inflammation and nerve damage and also provides symptomatic relief. Generally these agents are tapered and discontinued once the inflammation is controlled to prevent formation of cataracts and increased intraocular pressure. Steroids with decreased ocular side effects like fluorometholone and loteprednol are preferred.
- Non-steroidal anti-inflammatory therapies: Topical cyclosporine 0.5% has been shown to decrease the surface inflammation. Also a new drug –lifitegrast has been shown to decrease surface inflammation. These agents modulate T cell mediated inflammation.
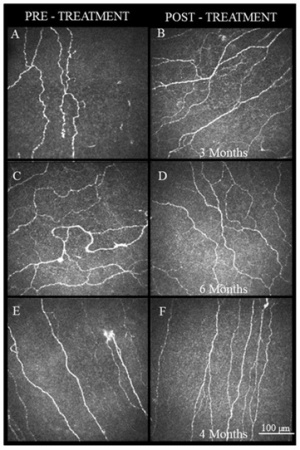
Neuro-regenerative therapy
Autologous serum tears – These therapies target the underlying pathophysiology of aberrant nerve regeneration subsequent to repeated injury in neuropathic pain. Various neurotrophic growth factors and epithelial growth factors such as the nerve growth factor (NGF), insulin-like growth factor-1, transforming growth factor β, fibronectin, substance P and epidermal growth factor, which help in proliferation, differentiation and maintenance ocular surface health, are present in high concentrations in serum tears.[35][36] NGF is present in high concentrations in serum compared to tears and plays an important role in nerve regeneration and restoration of function of nerves.[37][38][39][40][41] Serum tears are also effective in dry eye and neurotrophic conditions of the cornea.[42][43][44][45][46]Figure 4.
Systemic analgesics, anti-depressives and anti-psychotics
Studies have shown that patients with neuropathic pain also have non ocular symptoms of discomfort. This disease entity is a part of the systemic complex pain syndrome. The systemic pain intensity correlates with depression and PTSD correlate with the dry eye like symptoms. These patients are treated with tri-cyclic antidepressants (e.g., amytriptyline, nortriptyline), anti-convulsants (e.g. carbamazepine), NSAIDS, dronabinol, tramadol, gabapentin/pregabalin are also used with variable success.[9] [47]
Central pain modulation
These treatments have shown to be effective in chronic intractable pain with central sensitization.
- Electrical neurostimulation - These treatments use the principle of pain gating. Direct neuro-stimulation of the large diameter afferent fibers along the corneal pain pathway using percutaneously placed monopolar electrodes. Specifically, the area of trigeminocervical complex where the nociceptive corneal afferents in the spinal trigeminal tract synapses with the second order neurons in the area of the C1 C2 levels of the cervical cord is targeted. Direct stimulation inhibits the small diameter nociceptor terminals. However, this is technically challenging and can have complications as migration of the lead. Trans Magnetic Stimulation (TMS) has also been recommended for neuropathic pain.
- Intrathecal infusion of analgesics – Intrathecal infusion of fentanyl and bupivacaine at the cervical cord level has recently shown to have good long term pain relief. Complications include catheter tip granuloma formation.
Other
Vitamin B supplementation has been shown to be effective in herpes, diabetic neuropathy and neuropathic pain. This is because of the analgesic effects and anti-nociceptive effects. Vitamin B 12 topical therapy leads to re-innervation and re-epithelization of the corneal surface. Vitamin 12 acts through the blockade of the cyclooxygenase pathway. It has anti-nociceptive effects by increasing the serotonin levels in different regions of the brain, inhibiting the nociceptive neurons in spinal cord. Decreasing response of thalamic neurons, or by activating opioid receptors.[48] Vitamin D supplementation has also been shown to be effective in specific cases.[49] Cardiovascular exercise, dietary modification, meditation, and acupuncture have also been shown to be effective.[47]
Botulinum toxin A has also been shown to be a potential treatment. Patients who underwent BoNT-A treatments for chronic migraine also reported improvements in dry eye and photophobia symptoms, which may be explained by 1) convergence in nociceptive signaling, sensitization, and signal amplification in migraine, photophobia, and dry eye symptoms in the trigeminal cervical complex and posterior thalamus and 2) the role of inflammatory mediators such as calcitonin gene related peptide in both migraines and photophobia. [50]
Additional Resources
- Pain in Eye. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/symptoms/pain-in-eye-list. Accessed March 21, 2019.
- Galor A, Margolis TP. Ocular Pain: An Important Yet Under-Evaluated Feature of Dry Eye. Clinical Education Course. San Francisco: American Academy of Ophthalmology, 2018. https://www.aao.org/course/ocular-pain-course Accessed September 27, 2018.
References
- ↑ Jump up to: 1.0 1.1 Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or "phantom" cornea? J Refract Surg. 2007;23(6):598-602.
- ↑ Jump up to: 2.0 2.1 Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. 2009;7(1):28-40.
- ↑ Jump up to: 3.0 3.1 Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10(1):2-14.
- ↑ Jump up to: 4.0 4.1 Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2016.
- ↑ Jump up to: 5.0 5.1 Galor A, Covington D, Levitt AE, et al. Neuropathic Ocular Pain due to Dry Eye is Associated with Multiple Comorbid Chronic Pain Syndromes. J Pain. 2016;17(3):310-318.
- ↑ Jump up to: 6.0 6.1 Rosenthal P, Borsook D, Moulton EA. Oculofacial Pain: Corneal Nerve Damage Leading to Pain Beyond the Eye. Invest Ophthalmol Vis Sci. 2016;57(13):5285-5287.
- ↑ Jump up to: 7.0 7.1 Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. Ocul Surf. 2015;13(3):250-262.
- ↑ Jump up to: 8.0 8.1 Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond). 2015;29(3):301-312.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain-Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016;31(1-2):59-70.
- ↑ Jump up to: 10.0 10.1 Kalangara JP, Galor A, Levitt RC, Felix ER, Alegret R, Sarantopoulos CD. Burning Eye Syndrome: Do Neuropathic Pain Mechanisms Underlie Chronic Dry Eye? Pain Med. 2016;17(4):746-755.
- ↑ Jump up to: 11.0 11.1 11.2 Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128-134.
- ↑ Theophanous C, Jacobs DS, Hamrah P. Corneal Neuralgia after LASIK. Optom Vis Sci. 2015;92(9):e233-240.
- ↑ Jump up to: 13.0 13.1 Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521-542.
- ↑ Acosta MC, Tan ME, Belmonte C, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001;42(9):2063-2067.
- ↑ Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004;2(4):248-253.
- ↑ Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513-525.
- ↑ Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What Causes Eye Pain? Curr Ophthalmol Rep. 2015;3(2):111-121.
- ↑ Aicher SA, Hermes SM, Hegarty DM. Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis. Neuroscience. 2013;232:182-193.
- ↑ Bhave G, Gereau RWt. Posttranslational mechanisms of peripheral sensitization. J Neurobiol. 2004;61(1):88-106.
- ↑ Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51(2):240-264.
- ↑ Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1-3):184-187.
- ↑ Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32.
- ↑ Jump up to: 23.0 23.1 Stapleton F, Marfurt C, Golebiowski B, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on neurobiology. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS71-97.
- ↑ Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686-688.
- ↑ Seifert F, Maihofner C. Functional and structural imaging of pain-induced neuroplasticity. Curr Opin Anaesthesiol. 2011;24(5):515-523.
- ↑ Galor A, Zlotcavitch L, Walter SD, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol. 2015;99(5):665-668.
- ↑ Hamrah P, Qazi Y, Shahatit B, et al. Corneal Nerve and Epithelial Cell Alterations in Corneal Allodynia: An In Vivo Confocal Microscopy Case Series. Ocul Surf. 2016.
- ↑ Borsook D, Rosenthal P. Chronic (neuropathic) corneal pain and blepharospasm: five case reports. Pain. 2011;152(10):2427-2431.
- ↑ Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology. 2016.
- ↑ Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2016;100(6):745-749.
- ↑ Alzubaidi R, Sharif MS, Qahwaji R, Ipson S, Brahma A. In vivo confocal microscopic corneal images in health and disease with an emphasis on extracting features and visual signatures for corneal diseases: a review study. Br J Ophthalmol. 2016;100(1):41-55.
- ↑ Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Experiment Ophthalmol. 2009;37(1):100-117.
- ↑ Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2-15.
- ↑ Mian SZ, Agranat JS, Jacobs DS. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) Treatment for Complications After LASIK. Eye Contact Lens. 2016;42(6):371-373.
- ↑ Bradley JC, Bradley RH, McCartney DL, Mannis MJ. Serum growth factor analysis in dry eye syndrome. Clin Experiment Ophthalmol. 2008;36(8):717-720.
- ↑ Pan Q, Angelina A, Zambrano A, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2013;8:CD009327.
- ↑ Cirillo G, Cavaliere C, Bianco MR, et al. Intrathecal NGF administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol. 2010;30(1):51-62.
- ↑ Colangelo AM, Bianco MR, Vitagliano L, et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J Neurosci. 2008;28(11):2698-2709.
- ↑ Di Fausto V, Fiore M, Tirassa P, Lambiase A, Aloe L. Eye drop NGF administration promotes the recovery of chemically injured cholinergic neurons of adult mouse forebrain. Eur J Neurosci. 2007;26(9):2473-2480.
- ↑ Takemura Y, Imai S, Kojima H, et al. Brain-derived neurotrophic factor from bone marrow-derived cells promotes post-injury repair of peripheral nerve. PLoS One. 2012;7(9):e44592.
- ↑ Wiesmann C, de Vos AM. Nerve growth factor: structure and function. Cell Mol Life Sci. 2001;58(5-6):748-759.
- ↑ Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88(11):1467-1474.
- ↑ Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. Am J Ophthalmol. 2005;139(2):242-246.
- ↑ Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115-1120.
- ↑ Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. J Refract Surg. 2006;22(1):61-66.
- ↑ Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94(5):584-591.
- ↑ Jump up to: 47.0 47.1 Dieckmann G, Goyal S, Hamrah P. Neuropathic Corneal Pain: Approaches for management. Ophthalmology. 2017;124(11 Suppl):S34-S47.
- ↑ Shetty R, Deshpande K, Ghosh A, Sethu S. Management of Ocular Neuropathic Pain With Vitamin B12 Supplements: A Case Report. Cornea. 2015;34(10):1324-1325.
- ↑ Singman EL, Poon D, Jun AS. Putative corneal neuralgia responding to vitamin d supplementation. Case Rep Ophthalmol. 2013;4(3):105-108.
- ↑ Diel R, Kroeger Z, Levit R, Sarantopoulos C, Sered H, Martinez-Barrisonte J, Galor A. Botulinum toxin A for the treatment of photophobia and dry eye. Ophthalmology. 2018;125(1):139-140.