Ocular Manifestations of Xeroderma Pigmentosum
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder that occurs due to genetic defects in proteins involved in DNA repair. It is characterized by extreme ultraviolet light (UV) sensitivity with the development of skin cancer very early in life.[2] Patients with XP develop basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and cutaneous melanoma at an average age of 8 years old with a 10,000 fold increased risk for basal and squamous cell carcinomas and a 2000 fold increased risk for cutaneous melanomas.[2] One of four cases is associated with neurological symptoms including attenuated or missing deep tendon reflexes, speech and gait disturbances, cognitive decline, and progressive hearing loss.[3] Ocular manifestations are extremely common, shown to be present in 93% (83/89) of patients with XP in a study by Lim et al.[4] UV damage can quickly result in ocular cancer, making early recognition and diagnosis by an ophthalmologist crucial.[5]
Disease Entity
Epidemiology/Etiology
In the United States and Europe, incidence of XP is 1 in 1 million individuals. However, due to higher levels of consanguinity, incidence is much greater in Japan and north Africa, ranging from 1 in 22,000 to 1 in 100,000 individuals.[6] [7]
Risk Factors
Risk factors for development of disease include family history of XP as well as consanguinity due to the genetic nature of the disease.[7]
Pathophysiology
There are eight genetic groups of XP with differing symptoms and severity, each resulting from mutations in proteins with roles in repairing DNA damaged by UV radiation. The complementation groups XP-A to XP-G are associated with defects in nucleotide excision repair. There is 1 variant form, XP-V, that results from a mutation that inactivates DNA polymerase η. XP-A, XP-C, and XP-variant are the most common forms, comprising 30%, 27%, and 23.5% of cases, respectively.[6] The variation in clinical presentation of XP is explained by the number of complementation groups. For example, skin cancer has been found to be more common in complementation groups XP-C, XP-E, and, XP-V, and ocular damage most common in XP-C.[8]
Primary Prevention
There is no way to prevent the disease from occurring if one of the genetic mutations leading to XP is present.
Diagnosis
History
History indicating XP can include development of photophobia, sun damage, and skin cancer at an early age. Often patients may present with a blistering sunburn days after birth. However, half of XP patients have a non-burning phenotype and may tan instead. This half typically also has photophobia. Infants may cry and avoid looking at light sources.[9] Pigmentary skin changes often occur before 2 years of age with development of the first neoplasm at a median age of 8 years.[9][10]
Physical examination/Signs/Symptoms
The most common findings are cutaneous, ocular, and neurological. Cutaneous symptoms include erythema, swelling, blistering, and benign and malignant skin tumors including actinic keratosis, BCC, SCC, and malignant melanoma (MM).[9] Some of the earliest skin findings to look for include hyper or hypo-pigmented macules on sun-exposed skin, skin freckling before 2 years old, severe sun damage to the lips and eyes, and absence of skin lesions on sun-protected skin. Neurological symptoms are present in 17-25% of XP patients and include hearing loss, dysarthria, visual field disturbances, pes equinovarus, pes cavus, sensorineural hearing loss, diminished deep-tendon reflexes, acquired microcephaly, and optic neuropathy.[5] [9] [10] In a study of 89 patients with XP, Lim et al found neuro-ophthalmic abnormalities such as sluggish pupils (22/89), strabismus (7/89), and abnormal extraocular movements (6/89).[4]
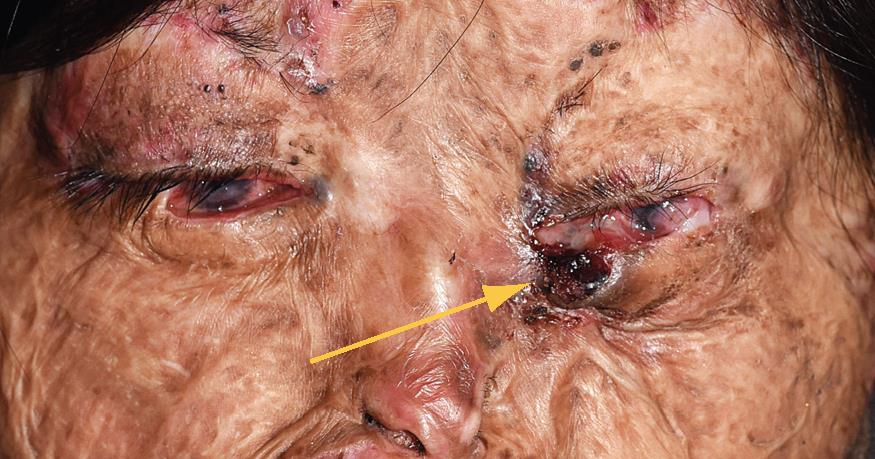
While there are a variety of systemic findings, we will focus on the ocular findings for this article. Lim et al found that 93% (83/89) of XP patients had at least one ophthalmic abnormality.[4] The anterior surfaces of the eyes (e.g. conjunctiva, cornea, sclera, eyelids, lens) are the most vulnerable to UV damage, while the posterior surfaces (e.g. retina) are more protected from UV light by the anterior tissues. However, there is rare development of choroidal melanoma in some XP patients.[11] Early ocular findings include conjunctival erythema, photophobia, pterygium, and corneal clouding.[9] Later signs include conjunctival xerosis, corneal drying, conjunctivitis, corneal cicatrization, ectropion, conjunctival pigmentation, and cataract.[9] Prolonged exposure can result in BCC and SCC of the eyelids and conjunctival melanoma.[9]
In a study of 87 patients with XP, Brooks et al found that the most common ocular abnormalities were conjunctivitis (51%), corneal neovascularization (44%), dry eye (38%), corneal scarring (26%), ectropion (25%), blepharitis (23%), conjunctival melanosis (20%), and cataracts (14%). Eyelid abnormalities included extropion (25%), blepharitis (23%), and lagophthalmos (10%).[12] 5 patients were found to have ocular neoplasms including SCC and MM, and 12 were found to have lid lesions including SCC and BCC.[12] Another study investigating ocular features in 209 patients with XP found that the most common presenting complaints were photophobia (197/418 eyes, 47.1%), ocular discomfort (179/418 eyes, 45.1%), and defective vision (153/418 eyes, 36.6%).[13] Ocular surface tumors were present in 44% (185/418 eyes) and eyelid tumors in 4% (18/418 eyes).[13] In multiple studies of patients with XP, conjunctival SCC was the most commonly found ocular surface malignancy while BCC was the most commonly found lid tumor.[13][14] [15]
Clinical diagnosis
Diagnosis of XP is suspected when the above signs and symptoms are present at an early age. Diagnosis is typically made clinically and confirmed with diagnostic procedures like genetic phenotyping listed below.[6]
Diagnostic procedures and Laboratory Testing
When concerned for XP, testing includes DNA analysis for XP gene mutations as well as functional testing including UV sensitivity, unscheduled DNA synthesis, and host cell reactivation.[9] For example, an MTT assay can discern post-UV survival of patient cells, and host cell reactivation tests the repair of a plasmid irradiated by UV light.[2] [6]
Differential diagnosis
Cockayne syndrome (CS), Trichothiodystrophy (TTD), Cerebrooculofacioskeletal syndrome (COFS), COFS/TTD, CS/TTD, UV sensitive syndrome, Rothmund-Thomson syndrome, Hartnup disorder, Carney complex.[5]
Management
General treatment
The mainstay of treatment for ocular manifestations of XP is prevention of sun damage. Patients must use sunscreen with sun protective factor (SPF) greater than 30, protective UV blocking clothing and sunglasses. When indoors, schools and houses are enhanced with windows that block UV penetration. Often, XP patients will carry UV meters with them to test the safety of new environments.[9]
Medical therapy
No causative therapy has been developed, but regular monitoring by an ophthalmologist for occurrence of new ocular and lid lesions is crucial. Medical therapies that have been tried for patients with XP include interferon alpha (IFN-a2b), 5-fluorouracil, and imiquimod.[6] Shah et al found that an XP patient with history of numerous previous ocular surface squamous neoplasia achieved tumor regression to 49% of the original tumor surface area with use of topical IFNa2b.[16] Ocular lubrication should be used regularly to treat dry eye.[9]
New treatments being explored include vismodegib, a hedgehog pathway inhibitor, to treat recurrent BCC after surgery [5], and a bacterial DNA repair enzyme T4 endonuclease V to reduce occurrences of actinic keratoses and BCC.[17] There have also been recent efforts to develop gene therapy, but none have yet reached the clinical stage.[18]
Surgery and Potential Complications
Progressive ocular lesions such as pterygium, pinguecula, pannus, and corneal clouding may require surgical intervention. Malignancies such as SCC, BCC, and MM also need to be surgically removed.[9] Care must be taken as eyelid surgery can result in lagophthalmos with potential for exposure keratopathy and corneal scarring.[9] Corneal transplants have even been used in some XP patients to treat decreased vision.[9] A recent study found that keratoplasty in 36 XP patients led to overall better vision outcomes, but there was graft failure in 15/54 eyes (35.7%). Most of the graft failures occurred due to graft rejection with vascularization or progressive scarring with vascularization.[19] Patients with XP should be monitored regularly for return of lesions after surgical removal, and adjuvant therapies like IFNa2b should be considered.
Prognosis
Patients can have a relatively normal lifespan if they are diligent with UV protective measures. Neurological abnormalities lead to progressive disabilities and shortened lifespan with median age of 29 years at death compared to 37 years at death for XP patients without neurological manifestations.[2] [5] The most common causes of death for XP patients include spread of skin cancer or internal cancer and neurological degeneration.[5] [10]
References
- ↑ Xeroderma pigmentosum. American Academy of Ophthalmology. https://www.aao.org/education/image/xeroderma-pigmentosum Accessed August 1, 2023.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Lehmann AR, McGibbon D, Stefanini M. Xeroderma pigmentosum. Orphanet J Rare Dis. 2011;6:70. doi:10.1186/1750-1172-6-70
- ↑ Anttinen A, Koulu L, Nikoskelainen E, et al. Neurological symptoms and natural course of xeroderma pigmentosum. Brain. 2008;131(Pt 8):1979-1989. doi:10.1093/brain/awn126
- ↑ Jump up to: 4.0 4.1 4.2 Lim R, Sethi M, Morley AMS. Ophthalmic manifestations of xeroderma pigmentosum. Ophthalmology. 2017;124(11):1652-1661. doi:10.1016/j.ophtha.2017.04.031
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 Kraemer KH, DiGiovanna JJ. Xeroderma pigmentosum. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle (WA): University of Washington, Seattle; June 20, 2003.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Lehmann J, Seebode C, Martens MC, Emmert S. Xeroderma pigmentosum - facts and perspectives. Anticancer Res. 2018;38(2):1159-1164. doi:10.21873/anticanres.12335
- ↑ Jump up to: 7.0 7.1 Nishigori C, Nakano E. Epidemiological study of xeroderma pigmentosum in Japan: genotype-phenotype relationship. In: Nishigori C, Sugasawa K, eds. DNA Repair Disorders. Springer Singapore; 2019:59-76. doi:10.1007/978-981-10-6722-8_5
- ↑ Fassihi H, Sethi M, Fawcett H, et al. Deep phenotyping of 89 xeroderma pigmentosum patients reveals unexpected heterogeneity dependent on the precise molecular defect. Proc Natl Acad Sci U S A. 2016;113(9):E1236-1245. doi:10.1073/pnas.1519444113
- ↑ Jump up to: 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 Tamura D, Ono R, DiGiovanna JJ, Kraemer KH. Management of xeroderma pigmentosum. In: Nishigori C, Sugasawa K, eds. DNA Repair Disorders. Springer Singapore; 2019:203-221. doi:10.1007/978-981-10-6722-8_14
- ↑ Jump up to: 10.0 10.1 10.2 Weon JL, Glass DA. Novel therapeutic approaches to xeroderma pigmentosum. Br J Dermatol. 2019;181(2):249-255. doi:10.1111/bjd.17253
- ↑ Vivian AJ, Ellison DW, McGill JI. Ocular melanomas in xeroderma pigmentosum. Br J Ophthalmol. 1993;77(9):597-598. doi:10.1136/bjo.77.9.597
- ↑ Jump up to: 12.0 12.1 Brooks BP, Thompson AH, Bishop RJ, et al. Ocular manifestations of xeroderma pigmentosum. Ophthalmology. 2013;120(7):1324-1336. doi:10.1016/j.ophtha.2012.12.044
- ↑ Jump up to: 13.0 13.1 13.2 Nandyala S, Mohamed A, Chaurasia S, Kaliki S, Ramappa M, Vemuganti GK. Ocular features in a large cohort of Indians with xeroderma pigmentosum. Cornea. 2021;40(5):571-577. doi:10.1097/ICO.0000000000002499
- ↑ Kaliki S, Jajapuram SD, Maniar A, Mishra DK. Ocular and periocular tumors in xeroderma pigmentosum: a study of 120 Asian Indian patients. Am J Ophthalmol. 2019;198:146-153. doi:10.1016/j.ajo.2018.10.011
- ↑ Alfawaz AM, Al-Hussain HM. Ocular manifestations of xeroderma pigmentosum at a tertiary eye care center in Saudi Arabia. Ophthalmic Plast Reconstr Surg. 2011;27(6):401-404. doi:10.1097/IOP.0b013e31821c7323
- ↑ Shah SU, Kaliki S, Kim HJ, Lally SE, Shields JA, Shields CL. Topical interferon alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on American Joint Committee on Cancer classification. Arch Ophthalmol. 2012;130(2):159-164. doi:10.1001/archophthalmol.2011.385
- ↑ Yarosh D, Klein J, O’Connor A, Hawk J, Rafal E, Wolf P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet. 2001;357(9260):926-929. doi:10.1016/s0140-6736(00)04214-8
- ↑ Warrick E, Garcia M, Chagnoleau C, et al. Preclinical corrective gene transfer in xeroderma pigmentosum human skin stem cells. Mol Ther. 2012;20(4):798-807. doi:10.1038/mt.2011.233
- ↑ Durgam S, Mohamed A, Ramappa M, Chaurasia S. Outcomes of keratoplasty in a cohort of Indian patients with xeroderma pigmentosum. Indian J Ophthalmol. 2021;69(4):860. doi:10.4103/ijo.IJO_2188_20