Ocular Manifestations of Phakomatoses (Neurocutaneous Syndromes)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Phakomatoses, also known as neurocutaneous syndromes, are a broad group of congenital disorders that are characterized by hamartomatous lesions of the skin and the central and peripheral nervous systems[1]. Initially, three conditions (neurofibromatosis, tuberous sclerosis, and von Hippel-Lindau) were described by Van der Hoeve, a Dutch ophthalmologist as “phakomatoses” by Van der Hoeve, a Dutch ophthalmologist, in order (Greek phakos: lentil, spot) to highlight the “lentiform” lesions that he observed in this group of disorders[2]. Later, he expanded the list to include Sturge-Weber syndrome. Ataxia-telangiectasia was added in 1941[3] and over time, more than 60 neurocutaneous syndromes have been described in the literature.
Originally, neurocutaneous syndromes were thought to originate from ectoderm, even though vascular anomalies seen in Sturge-Weber syndrome were known to be derived from mesoderm. Currently, many molecular and clinical pathologic features support the concept that the abnormal formation, migration, or differentiation of neural crest cells is the common pathogenesis of these syndromes[2]. Neural crest cells arise from embryonic ectoderm, and they give rise to a diverse cell population including structures of ectodermal origin (e.g., Schwann cells of the peripheral nervous system and dorsal root ganglia) and mesodermal origin (e.g., melanocytes, adipose tissue, connective tissue)[2][3][4]. The craniofacial abnormalities, angiomas, and skin lesions of mesodermal origin associated with many of the neurocutaneous syndromes emphasize that the common thread for these syndromes is due to abnormalities in neural crest cells.
In general, they share a molecular origin, where signaling pathways affect intra/extra neuronal function with phenotypic variability; some of them include mutations on PTEN, GNAQ, GNA11, RAS, MAPK/MEK, ERK, mTOR, RHOA, PI3K/AKT pathways
Causes
Neurofibromatosis
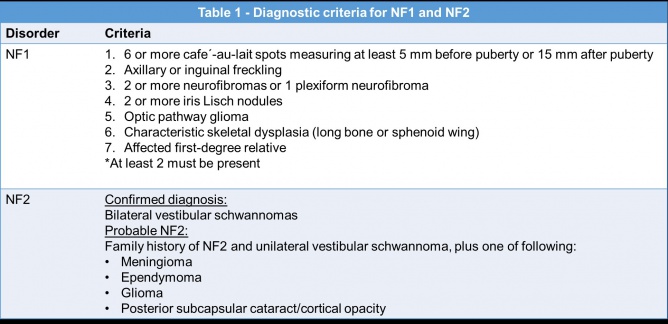
Neurofibromatosis is the most common phakomatosis. Although there are two types, neurofibromatosis type 1 (NF1) and neurofibromatosis type 2 (NF2)[5], the two disorders are distinct and in fact reside on different chromosomes. NF1 is more common than NF2 and affects approximately 1 in 3,000 individuals[6], while NF2 affects approximately 1 in 25,000 individuals worldwide[7]. Up to 50% of NF1 cases are autosomal dominant with complete penetrance and variable expressivity. The remainder of cases are de novo germ line mutations in the NF1 gene located on chromosome 17[8][9].Neurofibromin, the NF1 gene protein product, is a tumor suppressor expressed in various cells throughout the body, but especially in neurons, glial, Schwann cells, and melanocytes. The loss of this tumor suppressor leads to uncontrolled cell proliferation. In Schwann cells this manifests as neurofibromas, and in melanocytes as café au -lait macules and Lisch nodules in the iris. [10]
In contrast, NF2 is an autosomal dominant condition due to mutations in the NF2 gene located on chromosome 22. This produces a tumor suppressor protein product named merlin which similarly affects primarily schwann and leptomeningeal cells thus resulting in schwannomas, meningiomas, and ependymomas.[11] Similar to NF1, NF2 also has complete penetrance, variable expression, and a high rate of de novo mutations[7].
Tuberous Sclerosis
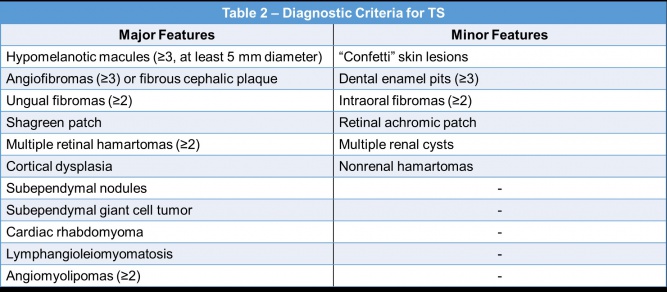
Tuberous sclerosis (TS) is also an autosomal dominant neurocutaneous genetic disorder that commonly involves the brain, skin, kidneys, heart, eyes, and lungs. Mutations in the genes of either TSC1 on chromosome 9 or TSC2 on chromosome 16[3][12] produce TS. TSC1 encodes the protein hamartin and TSC2 encodes the protein tuberin; both of these proteins are involved in the rapamycin (mTOR) pathway which functions to regulate cell growth and proliferation[12].
In contrast to NF, only 30% of the cases of TS are inherited in an autosomal dominant pattern and 70% of the TS cases are due to spontaneous mutations[13][14]. The incidence of TS is approximately 1 in 10,000 live births[15], however the true incidence may be higher because of undiagnosed mildly symptomatic individuals[3].
Sturge-Weber Syndrome
Sturge-Weber syndrome (SWS) is a sporadic congenital neurocutaneous syndrome that is characterized by a facial cutaneous hemangioma (port-wine stain) involving the skin in the trigeminal nerve territory[3][5]. The “port-wine stain” is often associated with an ipsilateral capillary-venous vascular malformation of the leptomeninges[9]. SWS has an estimated incidence between 1 in 20,000 and 50,000 live births[3][9]. It is hypothesized that SWS is the result of a somatic mutation in the GNAQ gene, with clinical manifestations differing depending on the timing and location of the mutation in the embryo[3]. Studies have suggested that port-wine stains that involve an entire V1 segment are commonly associated with underlying ophthalmic and neurologic involvement, and could be used as an initial sign to prompt further assessment[16].
Von Hippel-Lindau Disease
Von Hippel-Lindau (VHL) is an autosomal dominant condition characterized by vascular tumors of the retina, cerebellum, brainstem, and spine[9]. Although VHL is typically the result of a germline mutation of the VHL tumor suppressor gene on chromosome 3, De novo manifestation of VHL has been documented in up to 20% of newly diagnosed cases. [17]. The incidence of VHL is approximately 1 in 36,000 live births and there is almost 100% penetrance[17]. Individuals with the disease are characterized by the development of retinal and central nervous system hemangioblastomas, clear cell renal cell carcinomas, pheochromocytomas, pancreatic neuroendocrine tumors, and endolymphatic sac tumors.[18]
Ataxia Telangiectasia
Ataxia telangiectasia (AT) is an autosomal recessive condition that is caused by mutations in the ATM gene localized on chromosome 11, which normally plays a role in DNA damage repair[19]. The incidence of AT in the USA is approximately 1 in 88,000 live births, and symptoms typically present in infancy between the ages of 2 and 5[20]. The disease is characterized by cerebellar atrophy with progressive ataxia, cutaneous telangiectasias, higher risk to cancers (lymphoid malignancies), radiosensitivity, immune deficiency, recurrent sinopulmonary infections, and high levels of alpha-fetoprotein in serum[21]. Clinically patients can present with growth retardation, and premature aging to insulin resistance to neurodegeneration.[22]
Ocular Symptoms and Signs
Neurofibromatosis Type 1
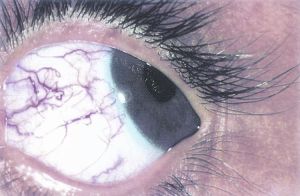
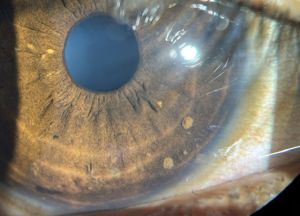
Lisch nodules are the most common ocular manifestation in NF1, and they are generally not found in patients with NF2[5]. Iris Lisch nodules are melanocytic hamartomas that are asymptomatic and tend to appear in late childhood[7]. They appear as small (<1-2 mm), dome-shaped, tan-dark brown pigmented on the iris, commonly on the inferior portion[3]. The prevalence of Lisch nodules is only 5% in children with NF1 under the age of 3, however the prevalence rises to 42% at the age of 3-4, 55% at ages 5-6, and 100% in adults over the age of 21[23].
Optic pathway tumors (e.g., gliomas) are common ophthalmic manifestations in NF1, and they occur in 5-25% of patients[24]. These are typically low-grade pilocytic astrocytomas that commonly involve the optic nerve[5]. These tumors can be isolated to a single optic nerve, or they can occur bilaterally and affect the optic chiasm[1]. Children with NF1 can also develop gliomas that are outside of the visual pathway (e.g., brainstem), and thus they present with cranial neuropathies, headaches, and additional neurological symptoms[24]. Common symptoms in patients with an optic pathway tumor include decreased visual acuity, loss of color vision, and visual field defects[9], however vision deficits only tend to occur in less than 50% of patients[25]. Patients with orbital involvement may have proptosis and/or strabismus.
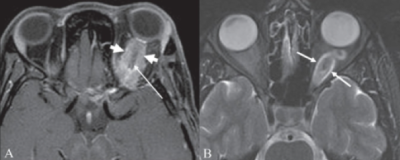
Plexiform neurofibromas are another characteristic symptom seen in patients with NF1. These are benign skin tumors that occur in less than 10% of patients with NF1, and they can involve the eyelid, eyebrow, orbit, and the temple[24]. These neurofibromas are soft and feel like “a bag of worms” upon palpation, and they present as a classic “S-shaped deformity” of the eyelid[5]. Consequences of these plexiform neurofibromas include proptosis, strabismus, amblyopia, congenital glaucoma, and skull deformities[3]. The lesions although benign can be extensive and cosmetically disfiguring[27]. Sphenoid wing dysplasia, an osseous abnormality resulting in thinning or absence of the greater sphenoid wing and may be associated with plexiform neurofibromas affecting the orbit[3][24].
Patients with NF1 are also at an increased risk of developing glaucoma, although it is an infrequent manifestation occurring in only 1-2% of NF1 patients[24][28][29]. Most of the cases of glaucoma are associated with ipsilateral plexiform neurofibromas of the upper eyelid[30].
Neurofibromatosis Type 2
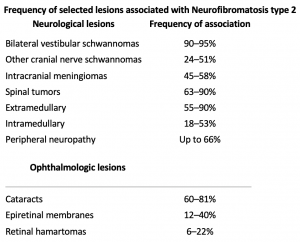
Ocular manifestations are much less common in NF2 than NF1. Optic nerve sheath meningiomas are a characteristic tumor type seen in NF2, in contrast to the optic pathway gliomas seen in NF1[31]. Other common findings include cataracts, hamartomas of the retina and retinal pigment epithelium, strabismus, amblyopia, and extra-ocular movement abnormalities[5][31]. NF2 is characterized by bilateral vestibular schwannomas. These lesions of the eighth cranial nerve can produce adjacent fifth, sixth, or seventh nerve dysfunction that can produce ocular symptoms and signs (e.g, facial numbness, pain, diplopia, exposure keratopathy).
Tuberous Sclerosis
The characteristic intraocular finding in TS is a retinal astrocytic hamartoma. These are typically benign lesions that can occur in up to 50% of patients, and they typically do not affect visual acuity unless they involve the macula or optic disc[3][12]. These lesions can manifest as three different morphological subtypes: (i) flat, smooth, translucent lesions without calcification; (ii) opaque, elevated, multinodular, “mulberry” lesions with calcifications; (iii) transitional lesions with features of both of the previous[3][32] Other ocular manifestations of TS include “punched out” areas of retinal depigmentation, palpebral angiofibromas, colobomas, and sectoral iris depigmentation[5][32].
Sturge-Weber Syndrome
Glaucoma is the most common ocular finding in SWS, with an occurrence in 30-70% of SWS patients[3][33]. Glaucoma can be unilateral or bilateral; unilateral glaucoma is typically associated with an ipsilateral port-wine stain[33]. Glaucoma that is congenital or develops within the first 2 years of life is commonly associated with corneal changes such as buphthalmos, corneal haze, and megalocornea[3][34]. In contrast, glaucoma that develops later in childhood usually results in painless, progressive visual field loss[35].
Choroidal hemangiomas (CH) are also common ocular manifestations in SWS that can occur in 20-70% of cases[33]. CH can be categorized as either diffuse or localized, and the diffuse form is most prevalent in patients with SWS[36]. These CH are typically found unilaterally and ipsilateral to the port-wine stain, although bilateral CH have been found with unilateral port-wine stains[37]. The CH often involve the posterior pole and fade into the periphery, or they can involve the entire fundus[5]. Some CH can lead to retinal complications and potential vision loss (e.g., retinal pigment atrophy, subretinal hemorrhage, serous retinal detachment, cystoid macular edema)[5][33].
Von Hippel-Lindau Disease
Retinal capillary hemangioma (RCH) is the hallmark intraocular manifestation of VHL disease. These RCH occur in 43-85% of patients with VHL disease[38]. The RCH appear as round, red lesions with dilated and tortuous vessels[5]. RCH may be variable in size and shape and can present in the periphery of the retina, the macula, or optic disc. RCH in VHL may be multifocal and/or bilateral[17]. The average age that patients present with a RCH is 25 years[17], and they are they are typically one of the earliest manifestations of VHL disease[39]. Most RCH are initially asymptomatic but can progress over time and lead to vision loss. Other RCH complications include retinal exudation, tractional retinal detachment, and neovascular glaucoma[40].
Ataxia Telangiectasia
Conjunctival telangiectasias are the most common ocular manifestations of AT[41]. Conjunctival telangiectasias usually occur by the age of 5-8 years old[42][43]. Visual acuity is typically unaffected in AT, however abnormalities in the control of eye movements (e.g., nystagmus, oculomotor apraxia, convergence and accommodation abnormalities, strabismus, and saccadic intrusions)[44][45] are common in AT.
Diagnosis
Neurofibromatosis Type 1
NF1 can be diagnosed with the clinical criteria defined in Table 1[7]. Genetic testing is possible but often reserved for patients with atypical presentations or those presenting with only one clinical feature[7][24].
Lisch nodules can be detected with a slit lamp examination, however they may be more difficult to detect in young patients especially when they are small or flat, or if they are located in iris crypts[39]. Lisch nodules are hamartomas Optical coherence tomography (OCT) of the anterior segment and ultrasound biomicroscopy can also be used to detect Lisch nodules[39].
Optic pathway glioma can present with loss in visual acuity, color alterations, and visual field alterations so a visual field examination can be important for screening for these tumors[39]. However, these tests can be difficult to perform on young children and vision deficits only occur in less than 50% of patients. Magnetic resonance imaging (MRI) is the best diagnostic technique to observe optic pathway tumors[9]. Several studies have also demonstrated thinning of the retinal nerve fiber layer with OCT in patients affected with optic pathway tumors[25][46][47].
Neurofibromatosis Type 2
NF2 is also diagnosed with clinical criteria (see Table 1), however the diagnosis of NF2 is sometimes more difficult than NF1 due to the lack of cutaneous symptoms[7]. Neuroimaging might show the characteristic bilateral vestibular schwannoma of NF2. Genetic testing is also available for NF2 and can detect more than 90% of mutations[7].
Tuberous Sclerosis
A diagnosis of TS can be made based on clinical features, genetic testing, or a combination of both[12]. The clinical criteria for the diagnosis of TS is displayed in Table 2[12][49]. A definite clinical diagnosis requires two major features or one major and two or more minor features[12].
The retinal astrocytic hamartomas can be diagnosed with a fundus examination, fluorescein angiography, or OCT[39]. The flat, smooth lesions can be difficult to identify during the fundus examination since they are translucent. The flat lesions are commonly located near a vessel, while the opaque, elevated lesions are peri- or epipapillary[39]. Fluorescein angiography is used to distinguish the flat, smooth lesions from the opaque, elevated lesions, and it can help recognize the vascularization[39]. Time domain OCT can be used to identify characteristics of the tumors and differentiate flat, smooth lesions from opaque, elevated lesions[50].
Pigment changes of the retina can be observed with a fundus examination or slit lamp examination. Palpebral angiofibromas can be observed with a slit lamp examination or an excisional biopsy. Colobomas can be diagnosed with ultrasound biomicroscopy or a fundus examination, and iris depigmentation can be diagnosed with a slit lamp examination[39].
Sturge-Weber Syndrome
A slit lamp examination can be used to diagnose most of the ocular manifestations found in SWS patients, however this exam may be difficult with young children[33]. Glaucoma progression is typically monitored with intraocular pressure (IOP) measurements, an optic nerve evaluation, and visual field testing[33]. Fundus examination with an indirect ophthalmoscope or biomicroscopy can be used to diagnose the classic “tomato ketchup” appearance of the CH[33][39]. There is also an increased number of well-formed choroidal vessels that can give the fundus a bright red appearance[5]. Enhanced depth spectral domain OCT imaging and MRI may also be useful to detect occult CH[33].
Von Hippel-Lindau Disease
Previously, the diagnosis of VHL was based on two criteria: a positive family history with one or more typical lesions, or no family history but two or more retinal lesions, or one retinal and one other visceral lesion. Now the definitive diagnosis of VHL is mainly done by gene analysis. Current molecular testing detects the VHL mutation in nearly 100 percent of those with the disease, which confirms the diagnosis.[51]
Ophthalmoscopy with a dilated eye can detect most retinal capillary hemangioblastomas[17]. Retinal hemangioblastomas will appear as globular, red-orange tumors with enlarged vessels that are located on the peripheral retina or near the optic disk[39]. Dilated fundoscopy exams should be performed once a year starting at the age of 1[17]. Fluorescein angiography can be useful to detect small peripheral hemangioblastomas that are difficult to detect with an ophthalmoscope[17][39]. OCT is also used to evaluate the small hemangioblastomas, but OCT is not useful in examining large lesions due to technical limitations of the device[52].
Ataxia Telangiectasia
The diagnosis of AT is mostly clinical, with common clinical features such as ataxia, telangiectasias, and abnormal eye movements and speech all supporting the diagnosis. In younger children, often the only presenting symptom is ataxia, as telangiectasias and eye movement abnormalities usually do not occur until later in the disease course. Thus, measurement of labs such as alpha fetoprotein level in the blood, elevated levels of CA125, and the absence of ATM protein on western blot can confirm the diagnosis. Physical exam findings such as conjunctival telangiectasias are essentially pathognomonic for AT. Additionally, oculomotor apraxia and abnormal ocular saccades are present in a majority of patients and should reinforce the diagnosis when present.[53] [54]. Observation of abnormal eye movements also help in the diagnosis of AT.
Treatment
Neurofibromatosis Type 1
Lisch nodules do not require treatment because they are benign and do not impair vision[3]. Children with NF1 are recommended to have annual evaluations by an ophthalmologist to monitor for optic pathway tumors by checking for visual acuity, visual fields, and color vision[55]. A MRI of the brain and orbit is typically only indicated if visual disturbances are detected[3] but some authors believe that neuroimaging screening for NF1 is an option. Progressive optic pathway tumors with visual loss may be treated with chemotherapy (e.g., vincristine or cisplatinum based regimens)[56]. Radiotherapy is an option as well but is not generally recommended for very young children because of potential neurologic complications and possible secondary malignancies that may result from the radiation[48]. Surgical removal of optic pathway gliomas is not generally feasible and can result in further visual morbidity. Cerebrospinal fluid shunting procedures may be necessary however for concomitant hydrocephalus.
Surgical removal of benign plexiform neurofibromas is often extremely challenging due to the location of the tumors and their vascular nature[48]. There is a high risk of life-threatening hemorrhage of facial tumors[24]. Agents such as mTOR inhibitors, fibroblast inhibitors, and inhibitors of kinases in the MAPK pathway are being investigated as potential therapies for plexiform neurofibromas, but evidence is currently insufficient to support the use of these treatments[3][57][58].
Tuberous Sclerosis
Retinal astrocytic hamartomas generally do not lead to visual loss and can be monitored with an annual ophthalmologic evaluation if baseline establishes presence of the hamartomas[59]. Some patients with TS have intratractable seizures for which vigabatrin may be prescribed[59][60]. Vigabatrin can cause retinal abnormalities, optic nerve atrophy, and visual loss and close ophthalmologic monitoring is generally recommended for these patients [61].
Sturge-Weber Syndrome
Both medical and surgical treatments of glaucoma have been performed to treat glaucoma in patients with SWS[33]. In early-onset SWS related glaucoma, medical therapy is generally initiated but surgical interventions are often required[3] (e.g., trabeculotomy and goniotomy)[33].
Port-wine stains on the face do not generally require therapy but can be successfully treated with laser for cosmetic/reconconstructive benefits[33]. While CH typically do not lead to visual impairment, they can lead to visual complications by causing macular edema and exudative retinal detachment[33]. Photodynamic[62][63] or other laser photocoagulation therapy can be used to treat symptomatic CH[33]. Alternative treatment options include external beam radiotherapy, brachytherapy, and anti-vascular endothelial growth factor (VEGF)[33].
Von Hippel-Lindau Disease
Early diagnosis and treatment of retinal hemangioblastomas is crucial to prevent visual loss or blindness[64]. Laser photocoagulation and cryotherapy are frequently used to treat peripheral retinal hemangioblastomas[64]. Tumors of the optic disc may be monitored without treatment due to the damage that treatments such as laser photocoagulation can cause[17]. VEGF-targeted therapies have been tested for tumors of the optic nerve, but the usefulness of this approach still needs to be defined[65][66][67].
Ataxia Telangiectasia
Conjunctival telangiectasias may be cosmetically bothersome but do not cause vision problems and are typically left untreated. Strabismus can be corrected with eye muscle surgery to improve the quality of life of patients with AT[43]. Drugs such as 4-aminopyridine have been prescribed to treat the eye movement disorders seen in patients with AT, but evidence of the usefulness of these drugs is not currently sufficient[43][68].
Summary
Clinicians should be aware of the ocular symptoms and signs of the phakomatoses. The ophthalmologist plays a critical role in both the diagnosis and treatment of the ocular manifestations of these disorders. A multidisciplinary approach to evaluation and management is critical in patients with phakomatoses in order to reduce or prevent the potentially vision or life threatening complications of these disorders.
References
- ↑ Jump up to: 1.0 1.1 Vézina,G. Neuroimaging of phakomatoses: overview and advances. Pediatr. Radiol.45 Suppl 3, S433-442 (2015).
- ↑ Jump up to: 2.0 2.1 2.2 Sarnat, H. B. & Flores-Sarnat, L. Embryology of the neural crest: its inductive role in the neurocutaneous syndromes. J. Child Neurol. 20, 637–643 (2005).
- ↑ Jump up to: 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 Chernoff, K. A. & Schaffer, J. V. Cutaneous and ocular manifestations of neurocutaneous syndromes. Clin. Dermatol. 34, 183–204 (2016).
- ↑ Gilbert, S. F. The Neural Crest. Dev. Biol. 6th Ed. (2000).
- ↑ Jump up to: 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 Woodward, L. M. Ocular manifestations of phakomatoses. Int. Ophthalmol. Clin. 54, 105–110 (2014).
- ↑ Friedman, J. M. Epidemiology of neurofibromatosis type 1. Am. J. Med. Genet. 89, 1–6 (1999).
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Korf, B. R. Chapter 39 - Neurofibromatosis. in Handbook of Clinical Neurology (eds. Dulac, O., Lassonde, M. & Sarnat, H. B.) 111, 333–340 (Elsevier, 2013).
- ↑ Cawthon, R. M. et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell 62, 193–201 (1990).
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 9.5 Nandigam, K., Mechtler, L. L. & Smirniotopoulos, J. G. Neuroimaging of neurocutaneous diseases. Neurol. Clin. 32, 159–192 (2014).
- ↑ Boyd, Kevin P et al. “Neurofibromatosis type 1.” Journal of the American Academy of Dermatology vol. 61,1 (2009): 1-14; quiz 15-6. doi:10.1016/j.jaad.2008.12.051.
- ↑ Asthagiri, Ashok R et al. “Neurofibromatosis type 2.” Lancet (London, England) vol. 373,9679 (2009): 1974-86. doi:10.1016/S0140-6736(09)60259-2.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 Randle, S. C. Tuberous Sclerosis Complex: A Review. Pediatr. Ann. 46, e166–e171 (2017).
- ↑ Jones, A. C. et al. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am. J. Hum. Genet. 64, 1305–1315 (1999).
- ↑ Au, K. S. et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet. Med. Off. J. Am. Coll. Med. Genet. 9, 88–100 (2007).
- ↑ Osborne, J. P., Fryer, A. & Webb, D. Epidemiology of tuberous sclerosis. Ann. N.Y. Acad. Sci. 615, 125–127 (1991).
- ↑ Ch’ng, S. & Tan, S. T. Facial port-wine stains –clinical stratification and risks of neuro-ocular involvement. J. Plast. Reconstr. Aesthet. Surg. 61, 889–893 (2008).
- ↑ Jump up to: 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 Lonser, R. R. et al. von Hippel-Lindau disease. Lancet Lond. Engl. 361, 2059–2067 (2003).
- ↑ Chittiboina, Prashant, and Russell R Lonser. “Von Hippel-Lindau disease.” Handbook of clinical neurology vol. 132 (2015): 139-56. doi:10.1016/B978-0-444-62702-5.00010-X.
- ↑ Teive, H. A. G. et al. Ataxia-telangiectasia -A historical review and a proposal for a new designation: ATM syndrome. J. Neurol. Sci. 355, 3–6 (2015).
- ↑ Alterman, N. et al. Ataxia-telangiectasia: mild neurological presentation despite null ATM mutation and severe cellular phenotype. Am. J. Med. Genet. A. 143A, 1827–1834 (2007).
- ↑ Riboldi GM, Samanta D, Frucht S. Ataxia Telangiectasia. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519542/
- ↑ Ambrose M, Gatti RA. Pathogenesis of ataxia-telangiectasia: the next generation of ATM functions. Blood. 2013;121(20):4036‐4045. doi:10.1182/blood-2012-09-456897.
- ↑ Lubs, M. L., Bauer, M. S., Formas, M. E. & Djokic, B. Lisch nodules in neurofibromatosis type 1. N. Engl. J. Med. 324, 1264–1266 (1991).
- ↑ Jump up to: 24.0 24.1 24.2 24.3 24.4 24.5 24.6 Kinori, M., Hodgson, N. & Zeid, J. L. Ophthalmic manifestations in neurofibromatosis type 1. Surv. Ophthalmol. 63, 518–533 (2018).
- ↑ Jump up to: 25.0 25.1 Avery, R. A. et al. Optic pathway glioma volume predicts retinal axon degeneration in neurofibromatosis type 1. Neurology 87, 2403–2407 (2016).
- ↑ Chavhan G. B., Shroff M. M., CC BY 2.0 <https://creativecommons.org/licenses/by/2.0>, via Wikimedia Commons https://commons.wikimedia.org/wiki/File:Optic_nerve_sheath_meningioma.png Accessed June 7, 2020.
- ↑ Avery, R. A. et al. Orbital/Periorbital Plexiform Neurofibromas in Children with Neurofibromatosis Type 1: Multidisciplinary Recommendations for Care. Ophthalmology 124, 123–132 (2017).
- ↑ Grant, W. M. & Walton, D. S. Distinctive Gonioscopic Findings in Glaucoma Due to Neurofibromatosis. Arch. Ophthalmol. 79, 127–134 (1968).
- ↑ Huson, S., Jones, D. & Beck, L. Ophthalmic manifestations of neurofibromatosis. Br. J. Ophthalmol. 71, 235–238 (1987).
- ↑ Greenwald, M. J. & Weiss, A. Ocular manifestations of the neurocutaneous syndromes. Pediatr. Dermatol. 2, 98–117 (1984).
- ↑ Jump up to: 31.0 31.1 Ardern-Holmes, S., Fisher, G. & North, K. Neurofibromatosis Type 2. J. Child Neurol. 32, 9–22 (2017).
- ↑ Jump up to: 32.0 32.1 Rowley, S., O’Callaghan, F. & Osborne, J. Ophthalmic manifestations of tuberous sclerosis: a population based study. Br. J. Ophthalmol. 85, 420–423 (2001).
- ↑ Jump up to: 33.00 33.01 33.02 33.03 33.04 33.05 33.06 33.07 33.08 33.09 33.10 33.11 33.12 33.13 Mantelli, F., Bruscolini, A., La Cava, M., Abdolrahimzadeh, S. & Lambiase, A. Ocular manifestations of Sturge-Weber syndrome: pathogenesis, diagnosis, and management. Clin. Ophthalmol. Auckl. NZ 10, 871–878 (2016).
- ↑ Stranzinger, E. & Huisman, T. a. G. M. [Sturge-Weber syndrome. Early manifestation and visualization of disease course]. Radiol. 47, 1126–1130 (2007).
- ↑ Awad, A. H., Mullaney, P. B., Al-Mesfer, S. & Zwaan, J. T. Glaucoma in Sturge-Weber syndrome. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 3, 40–45 (1999).
- ↑ Baselga, E. Sturge-Weber syndrome. Semin. Cutan. Med. Surg. 23, 87–98 (2004).
- ↑ Amirikia, A., Scott, I. U. & Murray, T. G. Bilateral diffuse choroidal hemangiomas with unilateral facial nevus flammeus in Sturge-Weber syndrome. Am. J. Ophthalmol. 130, 362–364 (2000).
- ↑ Wong, W. T. & Chew, E. Y. Ocular von Hippel-Lindau disease: clinical update and emerging treatments. Curr. Opin. Ophthalmol. 19, 213–217 (2008).
- ↑ Jump up to: 39.00 39.01 39.02 39.03 39.04 39.05 39.06 39.07 39.08 39.09 39.10 Abdolrahimzadeh, S., Plateroti, A. M., Recupero, S. M. & Lambiase, A. An Update on the Ophthalmologic Features in the Phakomatoses. J. Ophthalmol. 2016, (2016).
- ↑ Dollfus, H. et al. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Invest. Ophthalmol. Vis. Sci. 43, 3067–3074 (2002).
- ↑ Farr, A. K. et al. Ocular manifestations of ataxia-telangiectasia. Am. J. Ophthalmol. 134, 891–896 (2002).
- ↑ Cabana, M. D., Crawford, T. O., Winkelstein, J. A., Christensen, J. R. & Lederman, H. M. Consequences of the delayed diagnosis of ataxia-telangiectasia. Pediatrics 102, 98–100 (1998).
- ↑ Jump up to: 43.0 43.1 43.2 Rothblum-Oviatt, C. et al. Ataxia telangiectasia: a review. Orphanet J. Rare Dis. 11, 159 (2016).
- ↑ Shaikh, A. G. et al. Ataxia telangiectasia: a ‘disease model’ to understand the cerebellar control of vestibular reflexes. J. Neurophysiol. 105, 3034–3041 (2011).
- ↑ Shaikh, A. G. et al. Gaze fixation deficits and their implication in ataxia-telangiectasia. J. Neurol. Neurosurg. Psychiatry 80, 858–864 (2009).
- ↑ Parrozzani, R. et al. Optical coherence tomography in the diagnosis of optic pathway gliomas. Invest. Ophthalmol. Vis. Sci. 54, 8112–8118 (2013).
- ↑ Chang, L. et al. Optical coherence tomography in the evaluation of neurofibromatosis type-1 subjects with optic pathway gliomas. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 14, 511–517 (2010).
- ↑ Jump up to: 48.0 48.1 48.2 Ferner, R. E. et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet. 44, 81–88 (2007).
- ↑ Northrup, H. & Krueger, D. A. Tuberous Sclerosis Complex Diagnostic Criteria Update: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 49, 243–254 (2013).
- ↑ Mutolo, M. G. et al. OPTICAL COHERENCE TOMOGRAPHY AND INFRARED IMAGES OF ASTROCYTIC HAMARTOMAS NOT REVEALED BY FUNDUSCOPY IN TUBEROUS SCLEROSIS COMPLEX. Retina Phila. Pa 37, 1383–1392 (2017).
- ↑ Davis, Stephen, and Sami Uwaydat. “Diagnosis and Treatment of Von Hippel–Lindau Syndrome.” American Academy of Ophthalmology, 30 Mar. 2016. www.aao.org/eyenet/article/diagnosis-treatment-of-von-hippel-lindau-syndrome.
- ↑ Grigoropoulos, V. G., Nikolaidis, P., Emfietzoglou, I., Theodossiadis, P. G. & Theodossiadis, G. P. Evolution of a juxtapapillary von Hippel-Lindau tumour examined by optical coherence tomography. Clin. Exp. Optom. 95, 237–240 (2012).
- ↑ Gatti R, Perlman S. Ataxia-Telangiectasia. 1999 Mar 19 [Updated 2016 Oct 27]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26468/.
- ↑ Nissenkorn, A. & Ben-Zeev, B. Ataxia telangiectasia. Handb. Clin. Neurol. 132, 199–214 (2015).
- ↑ Cassiman, C., Legius, E., Spileers, W. & Casteels, I. Ophthalmological assessment of children with neurofibromatosis type 1. Eur. J. Pediatr. 172, 1327–1333 (2013).
- ↑ Packer, R. J. et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J. Neurosurg. 86, 747–754 (1997).
- ↑ Packer, R. J. et al. Plexiform neurofibromas in NF1: toward biologic-based therapy. Neurology 58, 1461–1470 (2002).
- ↑ Dombi, E. et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. N. Engl. J. Med. 375, 2550–2560 (2016).
- ↑ Jump up to: 59.0 59.1 Krueger, D. A., Northrup, H. & International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr. Neurol. 49, 255–265 (2013).
- ↑ Hébert-Lalonde, N. et al. Electrophysiological Evidences of Visual Field Alterations in Children Exposed to Vigabatrin Early in Life. Pediatr. Neurol. 59, 47–53 (2016).
- ↑ Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ. Characteristic retinal atrophy with secondary "inverse" optic atrophy identifies vigabatrin toxicity in children. Ophthalmology. 2004 Oct;111(10):1935-42. doi: 10.1016/j.ophtha.2004.03.036. PMID: 15465561; PMCID: PMC3880364.
- ↑ Tsipursky, M. S., Golchet, P. R. & Jampol, L. M. Photodynamic therapy of choroidal hemangioma in sturge-weber syndrome, with a review of treatments for diffuse and circumscribed choroidal hemangiomas. Surv. Ophthalmol. 56, 68–85 (2011).
- ↑ Patrianakos, T. D., Nagao, K. & Walton, D. S. Surgical management of glaucoma with the sturge weber syndrome. Int. Ophthalmol. Clin. 48, 63–78 (2008).
- ↑ Jump up to: 64.0 64.1 Singh, A. D., Nouri, M., Shields, C. L., Shields, J. A. & Perez, N. Treatment of retinal capillary hemangioma. Ophthalmology 109, 1799–1806 (2002).
- ↑ Schmidt, D., Natt, E. & Neumann, H. P. Long-term results of laser treatment for retinal angiomatosis in von Hippel-Lindau disease. Eur. J. Med. Res. 5, 47–58 (2000).
- ↑ Ziemssen, F., Voelker, M., Inhoffen, W., Bartz-Schmidt, K. U. & Gelisken, F. Combined treatment of a juxtapapillary retinal capillary haemangioma with intravitreal bevacizumab and photodynamic therapy. Eye Lond. Engl. 21, 1125–1126 (2007).
- ↑ Wong, W. T., Liang, K. J., Hammel, K., Coleman, H. R. & Chew, E. Y. Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease. Ophthalmology 115, 1957–1964 (2008).
- ↑ Shaikh, A. G. et al. Effects of 4-aminopyridine on nystagmus and vestibulo-ocular reflex in ataxia-telangiectasia. J. Neurol. 260, 2728–2735 (2013).