Ocular Manifestations of Huntington's Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Huntington’s disease is a genetic disease that usually presents in middle aged patients. It is due to a triplet repeat expansion in the IT15 gene.[1][2] A genetic test and diagnostic score on the Unified Huntington’s Disease Rating Scale are used to assist in making the diagnosis.[3] Eye symptoms associated with Huntington’s disease include ocular motility problems, like different characteristics of saccades, pursuit and fixation abnormalities, as well as retinal thinning.[4][5][6][7][8] Eye changes can occur early in Huntington’s, and research is ongoing regarding the utility of eye findings as potential biomarkers.[7][9][10] Whilst there is no formal treatment of Huntington’s disease, pridopidine is a drug that has the potential to help with some of the ocular manifestations of the disease.[11][12]
Disease Entity
Genetics
Huntington’s Disease (HD) is a genetic condition that has autosomal dominant inheritance.[13] The affected gene is IT15and is located at 4p16.3.[1][2] A (CAG)n expansion in the coding region codes to a polyglutamine repeat and a toxic protein, known as huntingtin.[1][2] The normal huntingtin protein, which is required for neuronal development, typically has about 35 or fewer triplet repeats, and fully penetrant disease manifests if more than 40 CAGs are present.[2][14] A range of 36-39 repeats leads to variation in penetrance.[14] Paternal transmission of the mutated gene is also correlated to a higher chance of juvenile-onset Huntington’s Disease.[15]
Epidemiology
The prevalence of HD varies across populations ranging from 5.7 per 100,000 in Europe and North America to a lower 0.4 per 100,000 in Asia.[16] The global prevalence is reported to be about 2.1 per 100,000.[16] The disease usually manifests in the fourth or fifth decade of life, but it can it also present in juveniles (<21 years old).[1] Generally, more CAG repeats correlate to earlier onset, yet the exact age of onset is too variable to predict by the triplet expansions alone.[13][17]
Diagnosis
There are two elements to diagnosis of HD.[3] First, a patient should either have a genetic test showing the CAG triplet expansion in the IT15 gene or a family history of the disease.[3] Secondly, “unequivocal signs of HD” outlined in the Unified Huntington’s Disease Rating Scale (UHDRS) should be observed in the patient.[3] Several ocular abnormalities are a part of the UHDRS and include ocular pursuit, saccade initiation, and saccade velocity; these are all tested both horizontally and vertically so that six items on the scale are eye-related.[18] A grade of 0 to 4 is given to each item on the scale - 0 corresponds to the patient not being affected and 4 to being affected most severely.[18] Other modalities testes in the UHDRS include oropharyngeal dysfunction, hand coordination, rigidity, bradykinesia, dystonia, chorea, gait, and balance – the score has 31 items, and a score of up to 124 is possible.[18]
Clinical Features
Saccades
Electro-oculographic recordings of patients with HD show abnormal or inability to perform vertical saccades with lower velocities, amplitudes and longer latencies.[4] These changes are not as distinct in horizontal saccades.[4] A study of 50 patients showed that saccadic abnormalities are common in HD patients, with 62% demonstrating slowed vertical saccades, 56% demonstrating a hypometric and reduced range of vertical saccades, and 89% demonstrating an increased latency.[5] Saccades can be further divided into reflexive (in response to novel stimuli) and voluntary (directly controlled).[4] HD patients often have problems with voluntary saccades - they have difficulty self-directing their eyes upon command.[4] In comparison, patients often have difficulty suppressing reflexive saccades, even when instructed to look away from a novel stimuli place in the visual field, which is thought akin to a ‘visual grasp’ reflex.[5] Patients with an early onset of the disease are characterized by saccadic slowing as the prominent symptom, though initiation (voluntary saccades) may not initially be affected, suggesting multiple contributing factors to saccadic abnormalities. [5]
Fixation and Pursuit Abnormalities
In HD patients, the balance between reflexive and voluntary saccades is distorted – the inability to appropriately initiate voluntary saccades means that reflexive saccades predominate.[4] This imbalance also manifests in fixation and pursuit abnormalities.[4] Fixation abnormalities refer to patients demonstrating irregular oscillations when focusing on a target.[4] The inability to smoothly pursue a moving target is another symptom, as patients demonstrate both jerky eye movement as a compensatory tactic and similar random oscillations.[4] Fixation and pursuit abnormalities are also common, with a majority of patients presenting with them.[5]
Retinal Thinning
A few recent papers have used optical coherence tomography to measure retinal thickness in HD patients and have obtained varying results.[6][7][8] One paper found that even though the average peripapillary retinal nerve fiber layer (RNFL) in patients was similar to that of the control group, the temporal RNFL was significantly thinner in HD patients at 62.3 um vs. the control group at 69.8 um.[6] Though the study could not test for progression, it did note that a longer disease duration showed a significant correlation with thinning.[6] A correlation between a higher UHDRS score and more thinning was noted, but it was not statistically significant.[6] A second study affirmed the presence of temporal RNFL thinning and also suggested that the superior RNFL could be similarly impacted.[8] A third study did not find either temporal or superior RNFL thinning and only observed a significant reduction of macular choroidal thickness.[7] Research into retinal thickness is relatively recent, and more studies are needed for conclusive findings.
Optic Nerve Degeneration
A 2022 study by Mazur-Michałek et al. examined the ultra-structural changes in the optic nerves of R6/2 mouse models using electron microscope imaging. The study revealed that the optic nerves of symptomatic R6/2 mice had lamellar separation of the myelin sheath, irregularly shaped and demyelinated axons, and the presence of myelonoid bodies. Quantitative analysis found that there was significant reduction in the thickness of the optic nerve myelin sheath in model mice compared to wild type mice [19]. These findings are novel, and more research is necessary to validate the results.
Eye Changes as Biomarkers
It is not currently possible to predict the age of onset of HD because of the lack of a marker - the length of CAG repeats does not always correlate to age of onset.[13][17] However, ocular abnormalities can be found in the pre-symptomatic stage of HD and studies suggest their severity may correlate with disease progression[20]. A more sensitive test that could objectively trace HD progression would make it easier to understand a therapy’s effectiveness.[9] The following are ocular findings which are being researched as potential biomarkers, although ongoing research is still necessary.
Saccade Latency
Measuring saccade latency may be a reliable and systematic biomarker for disease progression.[9][10] A study testing test-retest reliability found that saccade latency had an intraclass correlation coefficient of 0.55-0.87, which corresponds to moderate or high reliability.[10] Another study found that saccade latency could differentiate between patients who manifested HD motor signs and those patients who were premanifest.[9] Moreover, both groups of such patients had a regular 24 ms increase in latency over the 3-year study, suggesting the possibility of saccade latency as a biomarker.[9]
Macular Retinal Thickness
A study that found a decrease in macular retinal thickness also discovered a statistically significant regression equation that correlated average macular retinal thickness with a score on the UHDRS.[7] More research is needed to study both HD’s impact on macular retinal thickness and the sensitivity and specificity of this marker.
Pathophysiology of HD Manifestations
Saccades
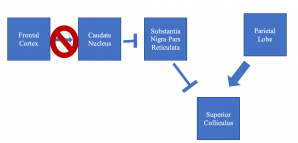
HD can cause region-specific neuronal loss in the brain, especially in the cortex and striatum.[21] Specifically, fewer connections are found between the frontal cortex and caudate nucleus in HD patients, and this pathway corresponds to the one used to initiate voluntary saccades but not reflexive ones.[22] Specific loss of neurons between these brain regions explains saccade initiation, pursuit, and fixation anomalies seen in HD.[22] The parietal lobe can still excite the superior colliculus to initiate voluntary saccades, but the degraded prefrontal-caudate nucleus tract prevents voluntary saccades due to a reduced ability to disinhibit the substantia nigra pars reticulata inhibition of the superior colliculus (see Figure 1).[23]
Retinal Thinning
Though the cause of retinal thinning is not well studied, one hypothesis suggests it may be related to disruptions in mitochondrial trafficking, given the presence of similar features in mitochondrial disorders.[8]Another recent theory suggests that the exchange of huntingin protein-containing myelinosomes between glial cells and retinal neurons may contribute to retinal damage.[24]
Management
Possible Therapy
Pridopidine is a dopaminergic stabilizer that is thought to potentially improve HD symptoms of ocular pursuit, saccade initiation, and velocity at a 90 mg/day dose.[11][12] A phase 3, randomized, double-blind, placebo-controlled trial found improvement in motor symptoms on the UHDRS, with statistically significant improvements in eye symptoms noted.[11] Further analysis is required into the potential benefits of this medication.
References
- ↑ 1.0 1.1 1.2 1.3 The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971-983. doi:10.1016/0092-8674(93)90585-E
- ↑ 2.0 2.1 2.2 2.3 Huang W-J, Chen W-W, Zhang X. Huntington’s disease: Molecular basis of pathology and status of current therapeutic approaches. Experimental and Therapeutic Medicine. 2016;12(4):1951-1956. doi:10.3892/etm.2016.3566
- ↑ 3.0 3.1 3.2 3.3 Reilmann R, Leavitt BR, Ross CA. Diagnostic criteria for Huntington’s disease based on natural history: Diagnostic Criteria of HD. Mov Disord. 2014;29(11):1335-1341. doi:10.1002/mds.26011
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Avanzini G, Girotti F, Caraceni T, Spreafico R. Oculomotor disorders in Huntington’s chorea. Journal of Neurology, Neurosurgery & Psychiatry. 1979;42(7):581-589. doi:10.1136/jnnp.42.7.581
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Lasker AG, Zee DS. Ocular motor abnormalities in Huntington’s disease. Vision Research. 1997;37(24):3639-3645. doi:10.1016/S0042-6989(96)00169-1
- ↑ 6.0 6.1 6.2 6.3 6.4 Kersten HM, Danesh-Meyer HV, Kilfoyle DH, Roxburgh RH. Optical coherence tomography findings in Huntington’s disease: a potential biomarker of disease progression. J Neurol. 2015;262(11):2457-2465. doi:10.1007/s00415-015-7869-2
- ↑ 7.0 7.1 7.2 7.3 7.4 Andrade C, Beato J, Monteiro A, et al. Spectral-Domain Optical Coherence Tomography as a Potential Biomarker in Huntington’s Disease: OCT in Huntington’s Disease. Mov Disord. 2016;31(3):377-383. doi:10.1002/mds.26486
- ↑ 8.0 8.1 8.2 8.3 Gatto E, Parisi V, Persi G, et al. Optical coherence tomography (OCT) study in Argentinean Huntington’s disease patients. International Journal of Neuroscience. 2018;128(12):1157-1162. doi:10.1080/00207454.2018.1489807
- ↑ 9.0 9.1 9.2 9.3 9.4 Antoniades CA, Xu Z, Mason SL, Carpenter RHS, Barker RA. Huntington’s disease: changes in saccades and hand-tapping over 3 years. J Neurol. 2010;257(11):1890-1898. doi:10.1007/s00415-010-5632-2
- ↑ 10.0 10.1 10.2 Blekher T, Weaver MR, Cai X, et al. Test–Retest Reliability of Saccadic Measures in Subjects at Risk for Huntington Disease. Invest Ophthalmol Vis Sci. 2009;50(12):5707. doi:10.1167/iovs.09-3538
- ↑ 11.0 11.1 11.2 de Yebenes JG, Landwehrmeyer B, Squitieri F, et al. Pridopidine for the treatment of motor function in patients with Huntington’s disease (MermaiHD): a phase 3, randomised, double-blind, placebo-controlled trial. The Lancet Neurology. 2011;10(12):1049-1057. doi:10.1016/S1474-4422(11)70233-2
- ↑ 12.0 12.1 Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for symptomatic treatment in Huntington’s disease. Cochrane Movement Disorders Group, ed. Cochrane Database of Systematic Reviews. Published online July 8, 2009. doi:10.1002/14651858.CD006456.pub2
- ↑ 13.0 13.1 13.2 Harper PS, Morris MR, Quarrell OWJ, Shaw DJ, Tyler A, Youngman S. Huntington’s disease. Major problems in neurology. Vol. 22. W.B. Saunders; London: 1991. The clinical neurology of Huntington’s disease; pp. 37–80.
- ↑ 14.0 14.1 Myers RH. Huntington’s disease genetics. Neurotherapeutics. 2004;1(2):255-262. doi:10.1602/neurorx.1.2.255
- ↑ van Dijk JG, van der Velde EA, Roos RAC, Bruyn GW. Juvenile Huntington disease. Hum Genet. 1986;73(3):235-239. doi:10.1007/BF00401235
- ↑ 16.0 16.1 Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Mov Disord. 2012;27(9):1083-1091. doi:10.1002/mds.25075
- ↑ 17.0 17.1 Stine OC, Pleasant N, Franz ML, Abbott MH, Folstein SE, Ross CA. Correlation between the onset age of Huntington’s disease and length of the trinucleotide repeat in IT-15. Hum Mol Genet. 1993;2(10):1547-1549. doi:10.1093/hmg/2.10.1547
- ↑ 18.0 18.1 18.2 Reilmann R, Schubert R. Motor outcome measures in Huntington disease clinical trials. In: Handbook of Clinical Neurology. Vol 144. Elsevier; 2017:209-225. doi:10.1016/B978-0-12-801893-4.00018-3
- ↑ Mazur-Michałek I, Kowalska K, Zielonka D, Leśniczak-Staszak M, Pietras P, Szaflarski W, Isalan M, Mielcarek M. Structural Abnormalities of the Optic Nerve and Retina in Huntington's Disease Pre-Clinical and Clinical Settings. Int J Mol Sci. 2022 May 13;23(10):5450. doi: 10.3390/ijms23105450
- ↑ Patel SS, Jankovic J, Hood AJ, Jeter CB, Sereno AB. Reflexive and volitional saccades: biomarkers of Huntington disease severity and progression. J Neurol Sci. 2012 Feb 15;313(1-2):35-41. doi: 10.1016/j.jns.2011.09.035. Epub 2011 Oct 21.
- ↑ Imarisio S, Carmichael J, Korolchuk V, et al. Huntington’s disease: from pathology and genetics to potential therapies. Biochemical Journal. 2008;412(2):191-209. doi:10.1042/BJ20071619
- ↑ 22.0 22.1 Klöppel S, Draganski B, Golding CV, et al. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington’s disease. Brain. 2008;131(1):196-204. doi:10.1093/brain/awm275
- ↑ 23.0 23.1 Tian JR, Zee DS, Lasker AG, Folstein SE. Saccades in Huntington’s disease: Predictive tracking and interaction between release of fixation and initiation of saccades. Neurology. 1991;41(6):875-875. doi:10.1212/WNL.41.6.875
- ↑ Yefimova MG, Béré E, Cantereau-Becq A, Meunier-Balandre AC, Merceron B, Burel A, Merienne K, Ravel C, Becq F, Bourmeyster N. Myelinosome Organelles in the Retina of R6/1 Huntington Disease (HD) Mice: Ubiquitous Distribution and Possible Role in Disease Spreading. Int J Mol Sci. 2021 Nov 25;22(23):12771. doi: 10.3390/ijms222312771

