Occult Macular Dystrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Occult macular dystrophy (OMD) is a rare dominantly inherited retinal degeneration characterized by a progressive decline in central vision with normal fundus. First described in 1989, this disease has become increasingly recognized.[1][2]The preponderance of literature has been published out of East Asia and it has been hypothesized to be more common in those of East Asian decent; however, due to the rarity of this disease and possible under-recognition, the epidemiology remains unknown. [3]
Etiology
OMD is typically described in the literature as an autosomal dominant disease caused by a mutation in the retinitis pigmentosa 1-like 1 (RP1L1) gene. However, only 50% of patients with OMD have a detectable genetic cause and there has been noted incomplete penetrance. Since the discovery of the association with RP1L1, most mutations reported are RP1L1 variants. The most common variant found is the c.133C>T, p.Arg45Trp mutation. [4][5][6][7][8][9][10][11] [12]
General Pathology
The RP1L1 gene has been found in both rods and cones. The precise function remains unknown, but based on the structural and electrophysiological characteristics in affected patients, it has been proposed the encoded protein is involved in the function and/or structure of outer photoreceptor segments. Cones seem to be affected earlier in the course of disease or in less severe cases, and in more advanced cases rod function in the macula is also affected.[13] It remains unknown why the disease primarily affects the fovea and why a more generalized cone dysfunction isn't present. RP1L1 mutations have also been associated autosomal recessive retinitis pigmentosa and cone dystrophy. [6][11][14]
Diagnosis
OMD is diagnosed by clinical history, retinal imaging and electrophysiological studies.
History
Patients present with a gradual visual acuity decline or a central scotoma. Symptom onset is variable, with a reported mean age of 25-30 and a range from 2 to 74.[5][11][15] Approximately 50% of patients will experience photophobia at some point during the course of their disease, while only a small minority experience light sensitivity at presentation. [15]
Physical examination
Visual acuity measurements are variable and likely depend on the stage or severity of disease. In the largest series published, the mean visual acuity was 20/80, with 20% having 20/30 or better vision and approximately 10% measuring 20/200 or worse. [3] Anterior and posterior segment exams are normal by definition of occult macular dystrophy. In certain pathogenic RP1L1 variants, there have been described clinically evidenced macular findings. These patients who progress to developing a more pronounced phenotypic presentation are no longer considered "occult" but are referred to has having RP1L1 maculopathy.[12]
Diagnostic procedures
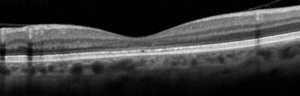
OCT plays an important role in both the diagnosis and monitoring progression of the disease. In general, the OCT reveals a blurring of the ellipsoid zone (EZ) and loss of the interdigitation zones (IZ) in the central macula (Figure 1), thinning of the outer nuclear layers in patients with severe disease and a preservation of the retinal pigment epithelium. [15][16][17]] The EZ can also appear thickened, blurred and dome-shaped in some patients. [15] Asymptomatic patients may show mild parafoveal abnormalities of the IZ and EZ. [18]
In reports there has been documented gradual changes in the OCT during the course of OMD. A study by Nakamura et. al, proposed three clinical stages based on the photoreceptor structures in the OCT images. Although not universally accepted yet at this time, these proposed clinical stages may be helpful in the investigation of the disease as well as monitoring its progression.[15]
Electroretinography demonstrates normal full-field cone and rod responses. OMD shows severe attenuation on macular or multifocal ERG(mfERG). Using mfERG , patients with occult macular dystrophy can be divided into 3 functional phenotypes: paracentral dysfunction, homogenous central dysfunction and widespread dysfunction. Paracentral dysfunction was noted to have milder phenotypes, while homogenous central or widespread dysfunction showed severe photoreceptor changes. [19]
Visual fields can reveal a central scotoma.
Fluorescein angiography is typically normal and some patients may have mild foveal hyperautofluorescence. [20]
Laboratory test
Genetic testing for known RP1L1 mutations can confirm the diagnosis and be helpful in ambiguous cases.
Classification
OMD can be classified into RP1L1-associated OMD (Miyake disease), other hereditary OMD caused by non-RP1L1 mutations and nonhereditary OMD.[21] There are subtle phenotypic differences between these groups, in particular nonhereditary OMD cases may not have involvement of both EZ and IZ. These phenotypic differences are suggestive of a possible differing mechanisms of photoreceptor injury and vision loss.[5][6][7][8][9][10][11]As mentioned previously, there has been cases with noted fundus/macular disruptions. These patients are classified as having RP1L1 maculopathy.[12]
Differential diagnosis
For patients with a normal appearing fundus and a vision decline, the differential diagnosis includes other retinal degenerations (such as cone dystrophy and early ABCA4-associated retinopathy), optic neuropathy, amblyopia, cortical vision loss and functional vision loss. Macular OCT, macular ERG or multifocal ERG in patients with unexplained visual acuity loss should be considered and can help in identifying patients with OMD.
Management
Currently no treatment is available for patients with OMD. Two major challenges in developing gene therapy for OMD, among many other challenges, are the rarity of disease and the unknown function of the RP1L1 gene. However, as gene therapy improves for other pathologies, OMD may eventually be a reasonable target because, unlike in many other inherited retinal degenerations, patients with OMD often present in adulthood, don't have amblyopia and appear have a large window of therapeutic intervention due to the slow predictable progression of disease.
Prognosis
The visual acuity for most patients appears to slowly worsen over a 10 to 15-year period and then remain stable. [5][18] However, significant variability exists between patients, and larger, long-term studies are needed to better counsel patients on their individual prognosis.
Additional Resources
Open access articles with examples of ERGs and additional OCT images can be found here:
Clinical Stages of Occult Macular Dystrophy Based on Optical Coherence Tomographic Findings
References
- ↑ Miyake Y, Ichikawa K, Shiose Y, Kawase Y. Hereditary macular dystrophy without visible fundus abnormality. Am J Ophthalmol. 1989;108(3):292-299. doi:10.1016/0002-9394(89)90120-7
- ↑ Miyake Y, Horiguchi M, Tomita N, et al. Occult macular dystrophy. Am J Ophthalmol. 1996; 122: 644–653.
- ↑ 3.0 3.1 Fujinami K, Yang L, Joo K, et al. Clinical and Genetic Characteristics of East Asian Patients with Occult Macular Dystrophy (Miyake Disease): East Asia Occult Macular Dystrophy Studies Report Number 1. Ophthalmology. 2019;126(10):1432-1444. doi:10.1016/j.ophtha.2019.04.032
- ↑ Akahori M, Tsunoda K, Miyake Y, et al. Dominant mutations in RP1L1 are responsible for occult macular dystrophy. Am J Hum Genet. 2010;87(3):424-429. doi:10.1016/j.ajhg.2010.08.009
- ↑ 5.0 5.1 5.2 5.3 Tsunoda K, Usui T, Hatase T, Yamai S, Fujinami K, Hanazono G, et al. Clinical characteristics of occult macular dystrophy in family with mutation of RP1L1 gene. Retina-J Retinal Vitreous Dis. 2012;32:1135–47
- ↑ 6.0 6.1 6.2 Kabuto T, Takahashi H, Goto-Fukuura Y, et al. A new mutation in the RP1L1 gene in a patient with occult macular dystrophy associated with a depolarizing pattern of focal macular electroretinograms. Mol Vis. 2012; 18: 1031–1039.
- ↑ 7.0 7.1 Hayashi T, Gekka T, Kozaki K, et al. Autosomal dominant occult macular dystrophy with an RP1L1 mutation (R45W). Optom Vis Sci. 2012; 89: 684–691.
- ↑ 8.0 8.1 Ahn SJ, Cho SI, Ahn J, Park SS, Park KH, Woo SJ. Clinical and genetic characteristics of Korean occult macular dystrophy patients. Invest Ophthalmol Vis Sci. 2013; 54: 4856–4863.
- ↑ 9.0 9.1 Davidson AE, Sergouniotis PI, Mackay DS, et al. RP1L1 variants are associated with a spectrum of inherited retinal diseases including retinitis pigmentosa and occult macular dystrophy. Hum Mutat. 2013; 34: 506–514.
- ↑ 10.0 10.1 Fujinami K, Kameya S, Kikuchi S, et al. Novel RP1L1 variants and genotype-photoreceptor microstructural phenotype associations in cohort of Japanese patients with occult macular dystrophy. Invest Ophthalmol Vis Sci. 2016; 57: 4837–4846
- ↑ 11.0 11.1 11.2 11.3 Zobor D, Zobor G, Hipp S, et al. Phenotype variations caused by mutations in the RP1L1 gene in a large mainly German cohort. Invest Ophthalmol Vis Sci. 2018; 59: 3041–3052
- ↑ 12.0 12.1 12.2 Noel NCL, MacDonald IM. RP1L1 and inherited photoreceptor disease: A review. Surv Ophthalmol. 2020 Nov-Dec;65(6):725-739. doi: 10.1016/j.survophthal.2020.04.005. Epub 2020 Apr 30. PMID: 32360662.
- ↑ Miyake Y, Tsunoda K. Occult macular dystrophy. Jpn J Ophthalmol. 2015;59(2):71-80. doi:10.1007/s10384-015-0371-7
- ↑ Kikuchi S, Kameya S, Gocho K, et al. Cone dystrophy in patient with homozygous RP1L1 mutation. Biomed Res Int. 2015; 2015: 545243.
- ↑ 15.0 15.1 15.2 15.3 15.4 Nakamura N, Tsunoda K, Mizuno Y, et al. Clinical Stages of Occult Macular Dystrophy Based on Optical Coherence Tomographic Findings. Invest Ophthalmol Vis Sci. 2019;60(14):4691-4700. doi:10.1167/iovs.19-27486
- ↑ Brockhurst RJ, Sandberg MA. Optical coherence tomography findings in occult macular dystrophy. Am J Ophthalmol. 2007;143(3):516-518. doi:10.1016/j.ajo.2006.10.025
- ↑ Koizumi H, Maguire JI, Spaide RF. Spectral domain optical coherence tomographic findings of occult macular dystrophy. Ophthalmic Surg Lasers Imaging. 2009;40(2):174-176. doi:10.3928/15428877-20090301-13
- ↑ 18.0 18.1 Kato Y, Hanazono G, Fujinami K, et al. Parafoveal Photoreceptor Abnormalities in Asymptomatic Patients With RP1L1 Mutations in Families With Occult Macular Dystrophy. Invest Ophthalmol Vis Sci. 2017;58(14):6020-6029. doi:10.1167/iovs.17-21969
- ↑ Yang L, Joo K, Tsunoda K, Kondo M, Fujinami-Yokokawa Y, Arno G, Pontikos N, Liu X, Nakamura N, Kurihara T, Tsubota K, Iwata T, Li H, Zou X, Wu S, Sun Z, Ahn SJ, Kim MS, Mun YS, Park KH, Robson AG, Miyake Y, Woo SJ, Sui R, Fujinami K; East Asia Inherited Retinal Disease Society Study Group. Spatial Functional Characteristics of East Asian Patients With Occult Macular Dystrophy (Miyake Disease); EAOMD Report No. 2. Am J Ophthalmol. 2021 Jan;221:169-180. doi: 10.1016/j.ajo.2020.07.025. Epub 2020 Jul 21. PMID: 32707201.
- ↑ Fujinami K, Tsunoda K, Hanazono G, Shinoda K, Ohde H, Miyake Y. Fundus autofluorescence in autosomal dominant occult macular dystrophy. Arch Ophthalmol. 2011;129(5):597-602. doi:10.1001/archophthalmol.2011.96
- ↑ Fujinami K, Kameya S, Kikuchi S, et al. Novel RP1L1 variants and genotype-photoreceptor microstructural phenotype associations in cohort of Japanese patients with occult macular dystrophy. Invest Ophthalmol Vis Sci. 2016; 57: 4837–4846.

