Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION) Associated with Air Travel
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Anterior ischemic optic neuropathy (AION) can be arteritic (due to giant cell arteritis most commonly) or non-arteritic. Most cases of AION are classified as non-arteritic anterior ischemic optic neuropathy (NAION), which is the most common cause of acute unilateral optic neuropathy in older individuals. NAION is presumed to be due to ischemia of the anterior portion of the optic nerve, but the precise pathophysiology remains controversial. The diagnosis of NAION is usually made clinically, and typical cases present with acute, unilateral, painless loss of vision, optic disc edema, and a relative afferent pupillary defect (RAPD) that leads to optic atrophy within a few weeks[1].
Risk Factors
Although vasculopathic risk factors (e.g., hypertension, diabetes, and systemic atherosclerosis) have been proposed as the cause of NAION, some cases remain unknown. Though hypoxia (e.g., obstructive sleep apnea) has been implicated in NAION and high-altitude environments—including air travel—may result in ischemia, the precise role of hypoxia in NAION associated with air travel remains ill-defined[2]. This EyeWiki article describes the possible role of air travel in NAION.
General Pathophysiology
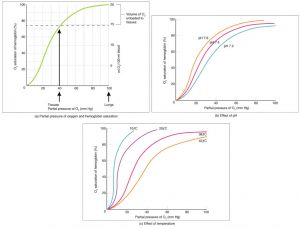
Two main mechanisms for the possible pathophysiology of NAION following air travel have been proposed[2][3][4]. The first and more likely mechanism is that of decreased oxygen saturation of the blood during air travel at high altitudes[3]. At higher altitudes, the partial pressure of oxygen is reduced, leading to a subsequent decrease in hemoglobin saturation of oxygen. Furthermore, the drop in oxygen availability triggers a right-shift in the hemoglobin-oxygen dissociation curve so that a decreased affinity for oxygen allows hemoglobin to rapidly release it to tissues in need[5]. Thus, travel at higher altitudes may lead to hypoxia through a decrease in oxygen saturation of hemoglobin.
Hemoglobin-Oxygen Dissociation Curve[6]
The second mechanism for NAION is through a thromboembolic event, whereby deep vein thrombi embolize and subsequently cause occlusion of the vessels supplying the optic nerve, producing ischemic optic neuropathy[3]. Formation of thrombo-emboli from the deep venous system are known to be associated with air travel; however, in order for a thrombo-embolus to trigger NAION, the formed thrombus must pass through a right-to-left shunt, such as a patent foramen ovale (PFO), to enter the arterial circulation and make its way to the short posterior ciliary vessels that supply the head of the optic nerve.
Air Travel
Commercial aircrafts counter the change in air pressure from lower altitudes (e.g., sea level) to higher altitudes by pressurizing their passenger cabins to equal the air pressure found at lower altitudes, known as the cabin altitude. The Federal Aviation Administration currently requires commercial aircrafts to maintain their cabin pressure equal to the pressure found at an altitude of 8,000 feet or lower (but not at sea level)[7]. The air pressure at 8,000 feet is thought to be sufficient for passengers and crew to fly comfortably without the need for supplemental oxygen. However, for a susceptible individual with vascular conditions or reductions in oxygen saturation at baseline, lower oxygen availability while flying may be a trigger for hypoxemia and thus, NAION[4].
For patients with a history of NAION in one eye, air travel in under-pressurized cabins may increase the risk of NAION in the contralateral eye[8]. Older generation aircraft and those with narrow bodies have been associated with less cabin pressurization as compared to newer generation and wider-bodied aircraft[4][8]. Additionally, cabin pressure has been shown to vary with flight duration. One study covering domestic air travel within the United States showed that flights shorter than 750 miles were associated with cabin pressures corresponding to an altitude of almost 2,000 feet lower than the cabin altitude on longer flights. If under 750 miles, the cabin altitude in these flights changed linearly based on distance, so that shorter flights had comparatively lower cabin altitudes, equating to decreased hypoxic stress[9].
Clinical Findings
The clinical findings for NAION following air travel are identical to those of NAION from any cause. These findings include: a RAPD of the affected eye; sectoral or generalized optic disc edema that progresses to optic disc pallor; visual field defects in accordance with the affected nerve fiber bundles, often altitudinal; and progression to thinning of the retinal nerve fiber layer of the site of involvement[10].
The subjective complaint typically comprises of an acute, painless, unilateral loss of vision that is variable and typically ranges from counting fingers to 20/20 but in rare occasions could even include no light perception (NLP) visual acuity[11]. The episode is not accompanied by typical symptoms and signs of giant cell arteritis, which would include scalp tenderness, headaches, jaw claudication, and high acute phase reactants such as ESR and CRP, distinguishing it from arteritic AION (AAION).
Reported Patient Cases
The following table outlines previously reported cases of NAION in patients following air travel or exposure to high altitude. Literature was reviewed through the National Library of Medicine, and search terms included “air-travel”, “non-arteritic anterior ischemic optic neuropathy", "flight", "high altitude", and "ischemic optic neuropathy".
| Patient Demographic | Past Medical Hisotry | Side of NAION Presentation | Information regarding flight or high altitude | Treatment |
|---|---|---|---|---|
| 41 year old male[2] | Seasonal allergies | OS | Male pilot presented after a flight in an A10 fighter jet while performing high G-force maneuvers | Not discussed |
| 52 year old male[3] | None | OS | 12 hour long airplane flight | Aspirin 200 mg/day |
| 48 year old male[12] | 10 pack-year history of cigarette smoking | OS | 15 hour long trans-Atlantic airplane flight in economy class | Aspirin 325 mg/day |
| 66 year old female[13] | Not discussed | OU | Following a trek at high altitude (>2500m [8202 feet]) | Not discussed |
| 33 year old male[14] | None | OD | Staying at Sjachen glacier (5472m [17,952 feet]) for 3 months | Not discussed |
| 12 year old male[15] | Bilateral disc drusen | OS | Following ascent in a hiking climb in Utah, USA and Colorado, USA | One dose of IV steroids initially
Dorzolamide/Timolol 0.5% drops OU |
The Austin Bradford Hill criteria includes nine aspects of association that, if met, may give more weight to inferring causality of NAION resulting from significant exposure to high altitudes[16]. These criteria include strength of association, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, and analogy. With the cases listed above, the first criteria of strength of association can be met, as altitude and subsequent development of NAION have been reported repeatedly. The diagnosis of NAION associated with high altitudes in a variety of age groups, situations, and with similar physical exam findings with either eye involvement shows consistency as well as specificity, meeting the second and third criteria. The somewhat immediate vision loss following high altitude exposure in each of the above cases suggests temporality.
With regards to a biological gradient, prior studies have suggested that longer exposure to higher altitudes likely contributes to greater hypoxemia, which is the basis for the proposed pathology[9]. The proposed pathophysiology aligns with the sixth and seventh criteria of plausibility and coherence. Experimentation, however, does rely on clear results without confounding variables, and is harder to accomplish. The final criteria of analogy may be demonstrated with comparison of high altitudes to systemic diseases such as obstructive sleep apnea, where decreased oxygen saturation puts the patient at risk, has been associated with NAION[2].
Differential diagnosis
Initial diagnosis involves differentiating the etiology of the AION as arteritic or non-arteritic, as the treatment regimen for AAION includes immediate administration of systemic corticosteroids to prevent involvement of the contralateral eye[17].
Management
Management for NAION initially involves differentiating it from AAION. Once this distinction has been made, control of vascular risk factors, such as through treatment of systemic hypertension or diabetes mellitus, is of utmost importance[1]. For the acute event and while working up to rule out AAION, steroids may be given; however, due to its side effect profile, the benefits and risks must be weighed for each individual patient to determine true efficacy[1][2]. The recommendation of high dose steroids until resolution of optic disc edema is thought to help relieve the pressure on the capillaries around the disc and allow circulation of the vessels supplying the optic nerve head[17]. However, other studies have not shown significant efficacy of this treatment in improving visual outcomes in NAION[18][19][20]. Finally, long-term use of vision aids should be used to augment any residual vision.
Prevention
Recognition of the possibility of NAION following air travel in individuals with vasculopathic and other risk factors may be the key to prevention. Systemic conditions such as obstructive sleep apnea, hypertension, severe anemia, and others should be medically managed and stable prior to flights. Although no clinical trials have been conducted, potential use of portable oxygen tanks for supplemental oxygen may aid in ascent and descent, as well as for any time cruising that a patient may feel distressed, especially on longer flights. As mentioned above, different types of aircraft have been associated with different cabin pressurizations. Thus, individuals at risk may choose to research their aircraft carriers prior to selecting a flight.
Summary
NAION is thought to result from vascular insufficiency to the anterior portion of the optic nerve. Patients at greatest risk often already have conditions contributing to hypoperfusion of the optic nerve, such as hypertension or obstructive sleep apnea. Air travel directly leads to decreased oxygen saturation in the blood at high altitudes despite cabin pressurization. This reduction of oxygen may contribute to ischemia of tissues, including decreased oxygen delivery to the short posterior ciliary vessels that supply the anterior portion of the optic nerve, thereby triggering NAION. Individuals with previous unilateral NAION have a 15% chance of a similar event in the contralateral eye in the next five years[21]. Although theoretically all passengers are at risk for NAION, those with this history of unilateral NAION, coupled with other underlying vascular insufficiencies, are at higher risks and should consider certain precautions if choosing to travel by air. Recognition of the association between NAION and air travel may be key in preventing the sequelae that follow.
References
- ↑ 1.0 1.1 1.2 Biousse V, Newman NJ. Ischemic Optic Neuropathies. N Engl J Med. 2015 Jun 18;372(25):2428-36. doi: 10.1056/NEJMra1413352. Erratum in: N Engl J Med. 2015 Dec 10;373(24):2390. PMID: 26083207.
- ↑ 2.0 2.1 2.2 2.3 2.4 Distefano AG, Lam BL. Non-Arteritic Anterior Ischemic Optic Neuropathy in Pilots. Aerosp Med Hum Perform. 2018 Nov 1;89(11):1005-1007. doi: 10.3357/AMHP.5177.2018. PMID: 30352654.
- ↑ 3.0 3.1 3.2 3.3 Panos GD, Panos LD, Hafezi F, Gatzioufas Z. Ischemic optic neuropathy after a long airplane flight: coincidence or rare economy class syndrome manifestation? Klin Monbl Augenheilkd. 2014 Apr;231(4):390-1. doi: 10.1055/s-0034-1368250. Epub 2014 Apr 25. PMID: 24771175.
- ↑ 4.0 4.1 4.2 Nazarali S, Liu H, Syed M, Wood T, Asanad S, Sadun AA, Karanjia R. Aircraft Cabin Pressurization and Concern for Non-Arteritic Anterior Ischemic Optic Neuropathy. Aerosp Med Hum Perform. 2020 Sep 1;91(9):715-719. doi: 10.3357/AMHP.5498.2020. PMID: 32867902.
- ↑ Lenfant C, Torrance JD, Reynafarje C. Shift of the O2-Hb dissociation curve at altitude: mechanism and effect. J Appl Physiol. 1971 May;30(5):625-31. doi: 10.1152/jappl.1971.30.5.625. PMID: 5572784.
- ↑ Anatomy & Physiology II. Module 6. https://courses.lumenlearning.com/suny-ap2/chapter/transport-of-gasesno-content/. Provided by: OpenStax CNX. Located at: http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@8.25.
- ↑ National Research Council (US) Committee on Air Quality in Passenger Cabins of Commercial Aircraft. The Airliner Cabin Environment and the Health of Passengers and Crew. Washington (DC): National Academies Press (US); 2002. Appendix C, Relevant Federal Aviation Regulations. Available from: https://www.ncbi.nlm.nih.gov/books/NBK207481/
- ↑ 8.0 8.1 Anna Ter-Zakarian, Rustum Karanjia, Alfredo A Sadun; Oxygen, Atmospheric Pressure and Non-arteritic Ischemic Optic Neuropathy. Invest. Ophthalmol. Vis. Sci. 2016;57(12):5088.
- ↑ 9.0 9.1 Hampson NB, Kregenow DA, Mahoney AM, Kirtland SH, Horan KL, Holm JR, Gerbino AJ. Altitude exposures during commercial flight: a reappraisal. Aviat Space Environ Med. 2013 Jan;84(1):27-31. doi: 10.3357/asem.3438.2013. PMID: 23304996.
- ↑ Foroozan R. New Treatments for Nonarteritic Anterior Ischemic Optic Neuropathy. Neurol Clin. 2017 Feb;35(1):1-15. doi: 10.1016/j.ncl.2016.08.003. PMID: 27886887.
- ↑ Luber, S., & Alweis, R. (2014). Keeping NAION visual loss: discriminating urgent versus emergent visual loss in an elderly female. BMJ case reports, 2014, bcr2013202262. https://doi.org/10.1136/bcr-2013-202262
- ↑ Kaiserman I, Frucht-Pery J. Anterior ischemic optic neuropathy after a trans-Atlantic airplane journey. Am J Ophthalmol. 2002 Apr;133(4):581-3. doi: 10.1016/s0002-9394(01)01371-x. PMID: 11931805.
- ↑ De Bats F, Gambrelle J, Feldman A, Mauget-Faysse M, Germain-Pastene M, Denis P. Survenue d'une neuropathie optique ischémique antérieure aiguë bilatérale en haute altitude: rôle de l'intolérance à l'hypoxie [Role of intolerance to hypoxia in the occurrence of anterior bilateral ischaemic optic neuropathy at high altitude]. J Fr Ophtalmol. 2010 Dec;33(10):724-7. French. doi: 10.1016/j.jfo.2010.09.009. Epub 2010 Nov 18. PMID: 21093103.
- ↑ Bandyopadhyay, Supratik MS; Singh, Ramandeep MS; Gupta, Vishali MS; Gupta, Amod MS Anterior Ischaemic Optic Neuropathy at High Altitude, Indian Journal of Ophthalmology: Volume 50 - Issue 4 - p 324-325
- ↑ Nanji AA, Klein KS, Pelak VS, Repka MX. Nonarteritic anterior ischemic optic neuropathy in a child with optic disk drusen. J AAPOS. 2012 Apr;16(2):207-9. doi: 10.1016/j.jaapos.2011.10.016. PMID: 22525184.
- ↑ Fedak, K. M., Bernal, A., Capshaw, Z. A., & Gross, S. (2015). Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerging themes in epidemiology, 12, 14. https://doi.org/10.1186/s12982-015-0037-4
- ↑ 17.0 17.1 Hayreh SS. Anterior ischaemic optic neuropathy. III. Treatment, prophylaxis, and differential diagnosis. Br J Ophthalmol. 1974;58(12):981–989.
- ↑ Rebolleda G, Pérez-López M, Casas-LLera P, Contreras I, Muñoz-Negrete FJ. Visual and anatomical outcomes of non-arteritic anterior ischemic optic neuropathy with high-dose systemic corticosteroids. Graefes Arch Clin Exp Ophthalmol. 2013 Jan;251(1):255-60. doi: 10.1007/s00417-012-1995-7. Epub 2012 Mar 24. PMID: 22441810.
- ↑ Pakravan M, Sanjari N, Esfandiari H, Pakravan P, Yaseri M. The effect of high-dose steroids, and normobaric oxygen therapy, on recent onset non-arteritic anterior ischemic optic neuropathy: a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2016 Oct;254(10):2043-2048. doi: 10.1007/s00417-016-3451-6. Epub 2016 Aug 10. PMID: 27510295.
- ↑ Pakravan M, Esfandiari H, Hassanpour K, Razavi S, Pakravan P. The Effect of Combined Systemic Erythropoietin and Steroid on Non-arteritic Anterior Ischemic Optic Neuropathy: A Prospective Study. Curr Eye Res. 2017 Jul;42(7):1079-1084. doi: 10.1080/02713683.2016.1270328. Epub 2017 Feb 26. PMID: 28632028.
- ↑ Newman NJ, Scherer R, Langenberg P, Kelman S, Feldon S, Kaufman D, Dickersin K; Ischemic Optic Neuropathy Decompression Trial Research Group. The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol. 2002 Sep;134(3):317-28. doi: 10.1016/s0002-9394(02)01639-2. PMID: 12208242.