Neuro Ophthalmic Complications of Immune Checkpoint Inhibitors
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
The typical manifestations of immune checkpoint inhibitor toxicity relate to autoimmune conditions of the involved structures, due to the mechanisms of ICI predisposing to overactivation of the immune system. Pembrolizumab is the most frequently associated ICI for neuro ophthalmic manifestations (~32% of cases in a large review)[1]. Neuro ophthalmologic manifestations are uncommon and it is important to consider the possibility that the manifestation is from the underlying malignancy or other treatments.
Overview
The use of immune checkpoint inhibitors (ICI) in treatment of malignancy has increased in prominence due to increasing research showing clinical benefit in multiple different malignancy types. This form of treatment, which involves activating the individual’s own immune system to target malignant cells, can sometimes cause unique adverse effects, which primarily relate to the activation or reactivation of autoimmune diseases. It is important to note however, that neuro ophthalmic manifestations may also be a direct result of the disease, or other treatments of the disease, and this should be taken into consideration as well.
Epidemiology
In one review of 109 patients from the literature, the overall incidence of neuro ophthalmic complications after ICI therapy was 0.46%[1]. Pembrolizumab was the most commonly associated ICI (32%), and cutaneous melanoma the most common indication for treatment (44%)[1]. Overal,l the rarity of neuro ophthalmic toxicities makes it challenging to further characterize risk factors / population factors that may predispose to these manifestations.
Mechanism of Action
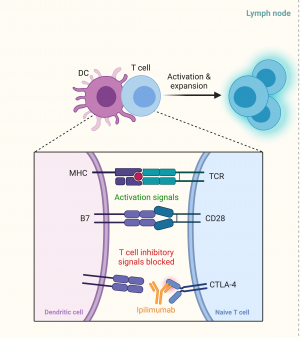
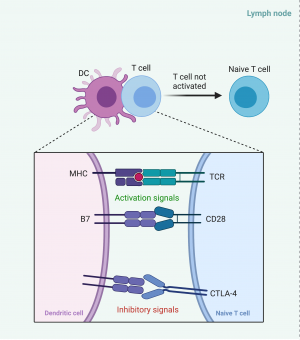
Immune checkpoints exist in the normal immune system to avoid the body mounting an immune system to self-antigens. The checkpoints exist within the lymph nodes, the ‘priming phase’, to stop activation of T lymphocytes in response to self-antigens, as well as in peripheral tissue, thein ‘effector phase’, to inhibit activated T lymphocytes responding to self-cells. T lymphocytes typically have a number of costimulatory molecules that need to be stimulated before being activated, but the inhibitory checkpoint mechanism can override these and stop T cell activation[2].
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a molecule found on T lymphocytes that can inhibit the activation of T cells. It typically binds to B7 on antigen presenting cells / dendritic cells within the lymph node. When the dendritic cell presents an antigen via a major histocompatibility complex (MHC) to a T cell receptor (TCR), this complex becomes an activating signal for the T lymphocyte. A second activating signal is the complex of B7 from the dendritic cell to the CD28 on the T cell. The combination of these two activating signals would normally lead to activation of the T cell (Figure 1a), however, the presence of a CTLA-4/B7 complex will inhibit this activation. CTLA-4 has a higher affinity and preferentially binds to B7 compared to CD28. Ipilimumab, a CTLA-4 inhibitor, will preferentially bind to the CTLA4 target on the T cell, stopping the inhibitory signal and causing activation of the T cells (Figure 1b) [2].
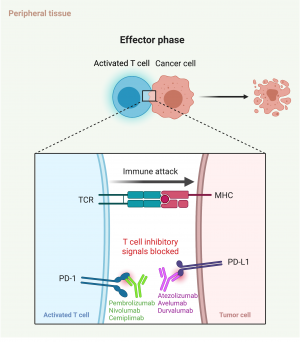
The complexing of programmed death 1 (PD1) and programmed death ligand 1 in the peripheral tissues similarly serves as an inhibitory signal to the activated circulating T cells (Figure 1c). Monoclonal antibodies to either the PD1 or the PD1 ligand can act to interrupt this binding, which stops the activated T cells from being inhibited (Figure 1d) .[2]
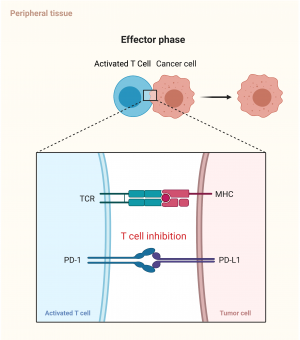
Because of the two different binding complexes, CTLA-4 inhibitors can be used in conjunction with PD-1 inhibitors to improve efficacy of cancer treatment[3].
The newest class of immune-checkpoint inhibitors are Lymphocyte activation gene-3 (LAG-3). LAG-3 is a a key member of the immunoglobulin superfamily (IgSF) locating on human chromosome 12, is a type I transmembrane protein detected to be expressed on the surface of effector T cells and regulatory T cells that participate in the regulation of T lymphocytes and antigen-presenting cells (APCs) signaling pathways and play a crucial part in the adaptive immune response. [4]Relatlimab is a monoclonal antibody against LAG-3 currently used only in combination with nivolumab to treat unresectable melanoma.
To date, there are 9 approved immune checkpoint inhibitors, targeting these 3 different molecules, as shown in the table below.
| Drug Class | Drug | Year First Approved | Clinical Indications (some for only refractory treatment or in combination with other medications) |
| CTLA-4 inhibitors | Ipilimumab | 2011[5] | Melanoma
Renal cell cancer Colorectal cancer Hepatocellular cancer Non-small cell lung cancer Malignant pleural mesothelioma |
| Tremelimumab | 2022[6] | Non-small cell lung cancer and small cell lung cancer
Hepatocellular cancer Biliary tree cancers | |
| PD-1 inhibitors | Pembrolizumab | 2014[7] | Melanoma
Non-small cell lung cancer Head and Neck Squamous Cell Cancer Classical Hodgkin lymphoma Primary mediastinal large B cell lymphoma Urothelial cancer Microsatellite instability-High or Mismatch Repair Deficient Cancer Gastric cancer Esophageal cancer Cervical cancer Hepatocellular cancer Merkel cell cancer Renal cell cancer Endometrial cancer Cutaneous squamous cell carcinoma Triple negative breast cancer |
| Nivolumab | 2014[8] | Melanoma
Non-small cell lung cancer Malignant pleural Mesothelioma Renal cell carcinoma Classical Hodgkin Lymphoma Head and neck Squamous cell carcinoma Urothelial carcinoma Colorectal cancer Hepatocellular carcinoma Esophageal cancer Gastric cancer | |
| Cemiplimab | 2018[9] | Cutaneous squamous cell carcinoma
Basal cell carcinoma Non-small cell lung cancer | |
| PD-L1 inhibitors | Atezolizumab | 2016[10] | Urothelial cancer
Non-small cell and small cell lung cancer Triple negative breast cancer Hepatocellular carcinoma Melanoma |
| Avelumab | 2017[11] | Merkel cell cancer
Urothelial carcinoma Renal cell carcinoma | |
| Durvalumab | 2017[12] | Non-small cell and small cell lung cancer | |
| LAG-3 inhibitors | Relatlimab | 2022[13] | Unresectable melanoma |
Because the aim of all of these agents is to activate the immune system, adverse effects are primarily related to overactivation of autoimmunity, which leads to multiple autoimmune manifestations (Figure 2).
Neuro ophthalmic manifestations
Multiple neuro ophthalmic manifestations have been reported in the literature, and are described below:
Optic neuropathy
ICI-associated optic neuropathy has typically been reported within the literature as either optic neuritis or non arteritic anterior ischemic optic neuropathy.
The standard presentation of optic neuritis is painful loss of vision unilaterally with pain on eye movements, altered color vision (dyschromatopsia), a relative afferent pupillary defect (RAPD) and no disc edema on examination, and optic nerve enhancement on magnetic resonance imaging (MRI). ICI-associated optic neuritis appears to present differently. Most cases of ICI associated optic neuritis have been associated with ipilimumab[1]. According to one retrospective review of 11 patients, only 10% of patients reported painful vision loss, 67% had dyschromatopsia, 36% were unilateral, and only 40% had abnormal MRI findings[14]. They also noted that being on multiple ICI was a risk factor for earlier onset optic neuritis (median onset cycle 4). The longest time to episode was 95 cycles[14]. Most cases of ICI associated optic neuritis have come from this single series of 18 episodes (11 eyes)[1][14]. There are two hypotheses for a difference in features of ICI associated optic neuritis – one is that the pathology is truly different to typical optic neuritis, and the other is that cases reported in the literature are mislabeled, and some of the ‘optic neuritis cases’ are actually non arteritic anterior ischemic optic neuropathy or other causes of optic neuropathy[1][14][15] – one review in fact excluded patients without typical optic neuritis features even when labelled as optic neuritis within the literature.
Orbital inflammatory syndrome
Orbital inflammatory syndrome refers to inflammatory diseases within the orbit, orbital apex, and the cavernous sinus. It is typically associated with autoimmune diseases, like most ICI complications. Clinical symptoms depend on the anatomic structures involved, which may include features of optic neuropathy (visual acuity loss, dyschromatopsia), eye pain, diplopia, proptosis. Examination findings may include features of optic neuropathy (decreased visual acuity, poor color vision, RAPD, optic disc edema), as well as ophthalmoplegia and proptosis. MRI will often be abnormal, with gadolinium enhancement in the affected regions. Orbital inflammatory syndromes have been described in isolated reports with multiple ICI[1].
Thyroid eye disease
Thyroid eye disease (TED) is an antibody mediated disease causing orbital fibroblast activation and resulting extraocular muscle enlargement without tendon enlargement. Patients will typically present with proptosis, diplopia, and eye pain. Examination findings may be significant for lid lag, lid retraction, exophthalmos, conjunctival injection. Typically the inferior and medial rectus muscles are involved early. Computerized tomography, MRI, or ultrasound of the orbits may show enlarged extraocular muscles without tendon involvement. TED occurs independent of thyroid hormonal status. The frequency of hormonal thyroid abnormalities has results in standard recommendations prior to ICI commencement of baseline thyroid function testing[16][17].
A meta-analysis of genetic polymorphisms found an association between the CTLA-4 +49A/G and TED. This could suggest a mechanism in which this disease is more prevalent in ICI, especially those with CTLA-4 targets[18]. Cases of TED have been seen in ipilimumab, pembrolizumab, and nivolumab[1].
Giant cell arteritis / temporal arteritis
Temporal arteritis is a large vessel vasculitis, with typical manifestations including temporal artery tenderness, jaw claudication, and vision loss (either transient or permanent). Other manifestations may relate to other large vessels affected, including aortitis, and ischemic stroke. Examination findings are dependent on the vessels affected, and may include features of optic neuropathy (decreased visual acuity, RAPD, optic disc edema), diplopia, temporal artery tenderness, or a weaker / absent temporal artery, or features of an ischemic stroke. ICI associated GCA has been reported with ipilimumab, nivolumab, and pembrolizumab, including some that are biopsy proven[1][19].
Myasthenia gravis
Myasthenia gravis (MG) is a systemic autoimmune disease of the neuromuscular junction, which typically presents with variable weakness and fatigue involving one or multiple systems. Ophthalmic manifestations may be related to purely ocular MG, or ocular features of generalized MG, and symptoms typically include painless and variable diplopia, ptosis, and / or ophthalmoplegia. Findings on examination may include a variable ptosis, Cogan’s lid twitch, and pseudo-retraction of the eyelid.
Cases of ICI associated ocular myasthenia gravis are yet to be reported in the literature, but large series of ICI associated generalized myasthenia gravis have noted an approximate prevalence of 0.24%, mostly associated with PD1 inhibitors[1] [20]. In the series, ptosis occurred in 75% of patients, and diplopia in 42% [20]. Median time to symptom onset was 29 days, although the longest case was noted 3 months after the last dose of ICI[1]
Management
The Common Terminology Criteria for Adverse Events (CTCAE) Version 5, published by the US Department of Health and Human Services has four grades for patients with ocular toxicity from medications[21]:
Grade 1: Mild toxicity (patients may be asymptomatic, but have clinically detectable findings)
Grade 2: Moderately symptomatic, which may interfere with ADLs and with visual acuity of 20/40 or better (or loss of 3 lines or fewer from baseline)
Grade 3: Decrease in vision (worse than 20/40, or more than 3 lines decreased from baseline, but better than 20/200), limiting activities of daily living, severe pain, and visual field defects
Grade 4: Visual acuity equivalent to or worse than 20/200
There are currently no formal treatment recommendations for neuro ophthalmic complications associated with ICIs. The Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group has suggested general guidelines for management of immune related adverse events by grade[16]:
Grade 1: Typically do not require corticosteroid administration or cessation of ICI.
Grade 2: Might consider temporary suspension of ICI and initiation of systemic corticosteroids (either intravenous or oral). Consider resuming ICI once symptoms have improved to grade 1.
Grade 3: Warrant consideration of suspension of ICI and cessation if symptoms haven’t resolved within 4 – 6 weeks, as well as systemic steroids.
Grade 4: Typically discontinue ICI and give systemic steroids.
A review of the literature noted that 62% of patients had their ICI discontinued due to the neuro ophthalmic complication[1]. In general, a risk: benefit analysis is required to ascertain the severity of the toxicity, in comparison to the benefit of the ICI, available alternative malignancy treatments, as well as the long term prognosis of the patient. Ocular adverse effects also rarely occur in isolation, and typically systemic adverse effects will also occur, making the decision to alter therapy likely to be multifactorial, and not always solely based on the specific ocular pathology[22].
The accepted treatments for the ocular condition when occurring separate to ICI administration are typically also considered for the respective ICI associated toxicity as well.
Optic neuropathy
Standard treatment for optic neuritis includes high dose corticosteroids, often followed by a rapid taper. Most (10 out of 11) patients in the largest review of ICI associated optic neuritis had their ICI ceased and received corticosteroids. Of the 16 eyes with poor visual acuity at onset of presentation, post-treatment vision improved, stabilized, and deteriorated in 12, 2, and 2 patients, respectively[14]. NAION management typically involves management of cardiovascular risk factors, and consideration of antiplatelet therapy.
Orbital Inflammatory syndrome
Corticosteroids are typically used, and specific treatment of the underlying cause is recommended (if identified). In patients with ICI associated orbital inflammatory syndrome, most patients have required systemic corticosteroids, and then typically additional therapies, including intravenous immunoglobulin, methotrexate, plasmapheresis, mycophenolate[1].
Thyroid eye disease
Treatment of TED can involve non-steroidal anti-inflammatory agents (NSAIDs), steroids, other immunosuppressive medications, or teprotumumab, a recently approved monoclonal antibody against insulin growth like factor [1]. Treatment typically depends on severity of symptoms, and will often start with the generally most tolerated medications (i.e. NSAIDs). Within the literature, most patients were treated with ICI associated TED were treated with systemic corticosteroids, although in this subgroup of patients, the risk/benefit profile of NSAIDs might be different, given higher risk of renal impairment, antiplatelet / anticoagulation / other contraindications to NSAID use1[1].
Giant cell arteritis
Standard treatment for suspected GCA involves urgent commencement of high dose corticosteroids as soon as the diagnosis is suspected. Given the significant morbidity associated with giant cell arteritis in the general population, all patients in the literature have been treated with systemic corticosteroids when this diagnosis was suspected / confirmed in association with ICI[19].
Myasthenia gravis
Standard treatment for myasthenia gravis is composed of acetylcholinesterase inhibitors (e.g. Pyridostigmine), as well as immunosuppressive therapies (mycophenolate, azathioprine, intravenous immunoglobulin, plasmapheresis). These two treatments domains are similar to what has been offered to patients with ICI-associated myasthenia gravis, although the longer term immunosuppressive agents are more frequently intravenous medication (corticosteroids, intravenous immunoglobulin, plasmapheresis). It should be noted that given the multi system involvement in myasthenia gravis, it is more likely the other system manifestations that would prompt discontinuation or suspension[1][20].
65 patients with ICI associated myasthenia gravis from a single center noted that treatments included steroids (94%), acetylcholinesterase inhibitors (51%), plasmapheresis (48%), intravenous immunoglobulin (44%) 17. Illness severity was marked (usually from non neuro ophthalmic manifestations), with 96% requiring hospitalization, and 19% requiring invasive ventilation[20].
Prognosis
Because of the relative infrequency of these toxicities, overall long term complications and prognosis are unknown. Some advocate routine ophthalmological examination every 4 – 6 months due to the possibility of ocular toxicities, however, given that specific treatment is not typically recommended for asymptomatic patients or grade 1 toxicities, this may not be of additional yield to the patient[23].
References
Acknowledgements: Figures created by authors with Biorender.com
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 Yu CW, Yau M, Mezey N, Joarder I, Micieli JA. Neuro-ophthalmic complications of immune checkpoint inhibitors: A systematic review. Eye and Brain. 2020;12:139-167. doi:10.2147/EB.S277760
- ↑ 2.0 2.1 2.2 Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Frontiers in Oncology. 2018;8(MAR). doi:10.3389/fonc.2018.00086
- ↑ Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2013;369(2):122-133. doi:10.1056/nejmoa1302369
- ↑ Huo, J.-L., Wang, Y.-T., Fu, W.-J., Lu, N., & Liu, Z.-S. (2022). The promising immune checkpoint LAG-3 in cancer immunotherapy: From basic research to clinical application. Frontiers in Immunology, 13. https://doi.org/10.3389/fimmu.2022.956090
- ↑ Yervoy (Ipilimumab) [Package Insert]. Bristol Myers Squibb; 2020.
- ↑ IMJUDO® (tremelimumab-actl) [Prescribing Information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2023.
- ↑ Keytruda (pembrolizumab) [package insert]. Merck Sharp Dohme; 2021.
- ↑ Opdivo (nivolumab) [package insert]. Bristol Myers Squibb; 2015.
- ↑ Libtayo (cemiplimab) [package insert]. Regeneron Pharmaceuticals; 2021.
- ↑ Tecentriq (atezolizumab) [package insert]. Genentech Inc; 2016.
- ↑ Bavencio (avelumab) [package insert]. EMD Serono Inc; 2017
- ↑ Imfinzi (durvalumab) [package insert]. Astrazeneca UK Ltd; 2021.
- ↑ Opdualag [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- ↑ 14.0 14.1 14.2 14.3 14.4 Francis JH, Jaben K, Santomasso BD, et al. Immune Checkpoint Inhibitor-Associated Optic Neuritis. Ophthalmology. 2020;127(11):1585-1589. doi:10.1016/j.ophtha.2020.05.003
- ↑ Micieli JA, Margolin E. Re: Francis et al.: Immune checkpoint inhibitor associated optic neuritis (Ophthalmology. 2020;127:1585-1589). Ophthalmology. 2020;127(11):e105-e106. doi:10.1016/j.ophtha.2020.05.043
- ↑ 16.0 16.1 Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. Journal for ImmunoTherapy of Cancer. 2017;5(1). doi:10.1186/s40425-017-0300-z
- ↑ Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: A review article. Current Opinion in Oncology. 2016;28(4):288-294. doi:10.1097/CCO.0000000000000296
- ↑ Wang H, Zhu LS, Cheng JW, et al. Meta-analysis of Association between the +49A/G Polymorphism of Cytotoxic T-Lymphocyte Antigen-4 and Thyroid Associated Ophthalmopathy. Current Eye Research. 2015;40(12):1195-1203. doi:10.3109/02713683.2014.993767
- ↑ 19.0 19.1 Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cellarteritis occurring in two patients after treatment withipilimumab, an antagonist of CTLA-4. Arthritis and Rheumatology. 2014;66(3):768-769. doi:10.1002/art.38282
- ↑ 20.0 20.1 20.2 20.3 Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: Single center experience and systematic review of the literature. Journal for ImmunoTherapy of Cancer. 2019;7(1). doi:10.1186/s40425-019-0774-y
- ↑ Common Terminology Criteria for Adverse Events (CTCAE). Version 5.; 2017.
- ↑ Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint Inhibitor Immune Therapy: Systemic Indications and Ophthalmic Side Effects. Retina. 2018;0(0):1-16.
- ↑ Vishnevskia-Dai V, Rozner L, Berger R, et al. Ocular side effects of novel anti-cancer biological therapies. Scientific Reports. 2021;11(1). doi:10.1038/s41598-020-80898-7