Neuro-ophthalmic Manifestations of Chronic Basilar Artery Occlusion
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease
Chronic basilar artery occlusion (CBAO) refers to a prolonged obstruction of the basilar artery, during which collateral circulation develops, mitigating the high mortality and morbidity typically associated with acute basilar artery occlusion.[1] CBAO can present in 3 ways: incidentally on angiography, with vertebrobasilar symptoms but without brainstem stroke, and with acute basilar artery occlusion without revascularization and a permanently occluded vessel for more than 3 months.[1]
Epidemiology
Posterior circulation strokes account for about 15% to 20% of all ischemic strokes but basilar artery occlusion (BAO) is only 1% to 4% of all ischemic strokes.[2]
Pathophysiology
The basilar artery starts at the medullopontine junction and terminates at the pons-midbrain junction. The basilar artery is split into three sections: proximal, middle, and distal. The basilar artery terminates as bilateral posterior cerebral arteries (PCA). Occlusive lesions can occur anywhere along the basilar artery and frequently manifest as pons ischemia. Most of the ischemia occurs in the paramedian base, but sometimes occasionally in the paramedian tegmentum. Atherosclerotic lesions are most common in the proximal and mid-basilar arteries, but thromboembolism and dissection are more common causes of posterior circulation large vessel ischemia including BAO. Top of the basilar syndrome and propagation of thrombus can be life threatening or can result in “locked in syndrome” where patients are awake and alert (intact cortex) but have lost all voluntary motor control (except sometimes the eye movements).
Blood vessel collateralization, which is commonly described in chronic occlusion, is the physiological and pathological process that involves the formation of collateral blood vessels as a compensatory mechanism in response to ischemia or vascular occlusion. Sufficient collateralization of the PCA is crucial in mitigating symptoms of chronic basilar artery occlusion.[1] Unlike acute occlusions, chronic occlusions can lead to milder symptoms due to the development of collateral blood flow.
CBAO in Neuro-ophthalmology
CBAO can result in either afferent or efferent visual pathway damage. In rare instances, both afferent and efferent symptoms arise from a single lesion. Afferent visual symptoms (e.g., homonymous hemianopsia, cortical blindness) are due to the posterior cerebral arteries (terminal arteries of the basilar artery) which supply the occipital lobes. While efferent visual symptoms (e.g., diplopia, nystagmus) can occur due to ischemia from the perforating arteries of the basilar trunk to the brainstem and cerebellum.
Diagnosis
Neurological Signs
For posterior large vessel strokes such as BAO, the latency period between the first prodromal symptoms and stroke onset can be days to months.[2] BAO can present with upper and lower extremity paresthesia as well as generalized weakness, difficulty walking, and light-headedness depending on the location of specific brainstem lesions.
Ocular Symptoms
The pons includes the sixth nerve nucleus and fascicle as well as the horizontal gaze pathways via the medial longitudinal fasciculus to the midbrain third nerve nucleus. Thus, ischemia to the efferent visual pathway of the brainstem can produce neuro-ophthalmic findings including unilateral or bilateral internuclear ophthalmoplegia (INO), One-and-a-half syndrome (a conjugate gaze palsy combined with an INO), and eight-and-a-half syndrome (One-and-a-half syndrome with seventh nerve palsy). In the afferent visual pathway, because the posterior cerebral arteries are the terminal branches of the basilary artery, the most frequent finding in BAO is homonymous hemianopia but cortical visual loss can also occur due to bilateral occipital ischemia. Occasional variations, such as occlusion of the anterior/posterior choroidal arteries can lead to homonymous sectoranopia, a wedge-shaped defect located near the horizontal meridian.
Diagnostic procedures
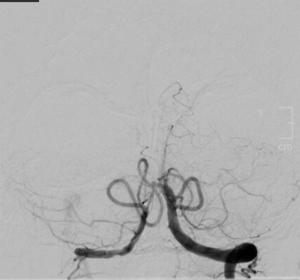
As with other ischemic changes, non-contrast head CT and CT angiography (CTA) are included in the initial workup of BAO. Vascular imaging (e.g., CTA) is a more sensitive method for determining the degree of stenosis and infarction in BAO. Diffuse weighted imaging (DWI) sequences in magnetic resonance imaging (MRI) of the head can demonstrate acute ischemic infarct. CT or MRI of the neck with CTA or MRA of the neck can demonstrate the occlusion as well as more proximal disease in BAO (e.g., vertebral artery dissection). Although CTA and MRA are often diagnostic in BAO, catheter digital subtraction angiography may be necessary in some cases.[3]
Differential diagnosis
The differential diagnosis is broad and encompasses both cortical and brainstem lesions that produce either efferent or afferent symptoms, these include but are not limited to:
- Foville Syndrome
- Millard Gubler Syndrome
- Subarachnoid Hemorrhage
- Intracranial Hemorrhage
Management
There is no consensus on the best acute management of BAO, but it is generally treated similarly to large vessel occlusive disease in other regions. The benefits of endovascular treatment for anterior circulation large-vessel occlusion (LVO) is well studied and established; however, there are still significant gaps for posterior LVO.[4] For BAOs, a timely acute stroke evaluation is critical, and treatment options such as thrombolysis or thrombectomy should be considered if the patient is within the treatment window. Although both intra-arterial thrombolysis (IAT) and intravenous thrombolysis (IV t-PA) have been used in BAO, one meta-analysis study of 420 patients with BAO showed no difference in outcomes between patients treated with IAT or IVT.[5] Mechanical thrombectomy has shown promising results among patients with BAO who presented 6 to 24 hours after symptom onset.[6] Acute and complete BAO typically leads to morbidity and possible long-term disability or death in the absence of spontaneous or post-treatment vascular recanalization. Some patients however have sufficient collaterals to allow alternate routes of perfusion to critical structures in chronic BAO. After a complete stroke evaluation including embolic and cardiogenic etiologies, the decision for antiplatelet (e.g., aspirin or dual platelet therapy) or anticoagulation will likely rest with a multidisciplinary team that includes the primary care physician and stroke neurology. Effective management of treatable vascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, achieving a healthy weight, and quitting smoking can improve outcomes and may even be lifesaving in some cases.
Prognosis
Acute and complete BAO in the past was associated with extremely high mortality (up to 90%) and high morbidity (e.g., pontine “locked-in” syndrome). However, more recent evaluation and treatments have improved the prognosis for BAO. Improved neuroimaging techniques, access to stroke centers, faster recognition of posterior stroke symptoms, and better treatment options (e.g., thrombolysis) have improved outcomes in BAO.
Summary
Although most patients with acute BAO have other neurologic findings that suggest posterior fossa ischemia, BAO can produce a wide range of neuro-ophthalmologic symptoms and signs affecting both the efferent and afferent visual pathways. These include lesions of the abducens nucleus (horizontal gaze palsy), ipsilateral or bilateral MLF (unilateral or bilateral INO), and facial nucleus/fascicular lesion (cranial nerve VII palsy). The combination of these efferent abnormalities with afferent visual pathway complaints (e.g., homonymous hemianopsia, cortical blindness) is suggestive of vertebrobasilar disease including BAO. Prompt and aggressive stroke evaluation and timely thrombolytic therapy or other emergent neuroradiological interventions can be vision, brain, or lifesaving.
References
- ↑ Jump up to: 1.0 1.1 1.2 Roth C, Yavuz R, Maschita C, Ferbert A, Matthaei J. Chronic basilar artery occlusion: a retrospective monocentric study. J Neurol. 2024 Jul;271(7):4423-4429. doi: 10.1007/s00415-024-12375-4. Epub 2024 Apr 27. PMID: 38676723.
- ↑ Jump up to: 2.0 2.1 Demel SL, Broderick JP. Basilar Occlusion Syndromes: An Update. The Neurohospitalist. 2015;5(3):142.
- ↑ Puetz V, Sylaja PN, Hill MD, et al. CT Angiography Source Images Predict Final Infarct Extent in Patients with Basilar Artery Occlusion. AJNR Am J Neuroradiol. 2009;30(10):1877.
- ↑ Ahmed RA, Dmytriw AA, Patel AB, Stapleton CJ, Vranic JE, Rabinov JD, Leslie-Mazwi TM, Rost NS, Hirsch JA, Regenhardt RW. Basilar artery occlusion: A review of clinicoradiologic features, treatment selection, and endovascular techniques. Interv Neuroradiol. 2023 Dec;29(6):748-758. doi: 10.1177/15910199221106049. Epub 2022 Jun 12. PMID: 35695210; PMCID: PMC10680956.
- ↑ Lindsberg PJ, Mattle HP. Therapy of Basilar Artery Occlusion. Stroke. 2006;37(3):922-928.
- ↑ Jovin TG, Li C, Wu L, et al. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. N Engl J Med. 2022;387(15):1373-1384.