Neuro-ophthalmic Manifestations in Adults after Childhood Periventricular Leukomalacia
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Neuro-ophthalmic Manifestations in Adults after Childhood Periventricular Leukomalacia
Disease
Periventricular Leukomalacia (PVL) is a condition characterized by injury to white matter adjacent to the ventricles of the brain. Premature birth is a strong risk factor for PVL. However, neuro-ophthalmic presentation of PVL is highly variable. Incidence of PVL in premature neonates is estimated to range from 8% to 22% 1,2; the cystic form of PVL, which is associated with more severe defects, has an estimated incidence of 5%3. PVL involving the optic radiations is one of the most common causes of visual impairment in patients with a history of prematurity4. The features of PVL in childhood are described elsewhere and this article will review the neuro-ophthalmic features of PVL in adult patients who may present later in life with otherwise unexplained findings that may mimic adult strabismus or optic neuropathy .
Etiology
Because the vascular supply of the periventricular region of the brain in utero remains immature late into term, PVL may arise from neonatal hypoglycemia, hypoxia, seizure, or infection in the third trimester or perinatally5,6. Cerebral visual impairment in PVL typically occurs because of afferent visual pathway injury to the optic radiations, which travel adjacent to the lateral ventricles7.
Risk Factors
Common risk factors for PVL are intrauterine infection, dysregulation of cerebral blood flow, chorioamnionitis, and premature membrane rupture6. However, the strongest and most direct risk factor for PVL is perinatal hypoxia8.
Pathophysiology
At the cellular level, hypoxia-ischemia results in inadequate neuronal and astrocyte glutamate uptake and consequent excitotoxicity. Reperfusion of ischemic tissue is associated with vascular injury, increased reactive oxygen and nitrogen species production, and abnormal inflammation5,8. Alternately, inflammatory cytokine response to bacterial infection as well as toxin from infection may injure susceptible oligodendrocyte precursors8. Consequent cell death and thinning of white matter around the ventricles occurs9. The resulting condition is therefore termed PVL. Although PVL is well known among pediatric ophthalmologists, adults may present with neuro-ophthalmic findings of childhood PVL.
PVL with ocular involvement typically includes characteristic pseudoglaucomatous nerve cupping. The cupping is a result of nerve cell atrophy following damage to the axons that synapse with them. This phenomenon is believed to result from transsynaptic degeneration and has been noted to be associated with the lateral geniculate nucleus pathology in pediatric populations. The optic radiations, which may be damaged in the periventricular region, originate from the lateral geniculate nuclei and are topographically organized both anatomically and functionally. Consequently, functional defects in patients with PVL are highly dependent on location of insult. Pre-chiasmal defects are usually associated with ipsilateral, loss of visual acuity or visual field deficit, dyschromatopsia, a relative afferent pupillary defect (RAPD) in unilateral or bilateral but asymmetric cases and optic atrophy in one or both eyes. In contrast, post-chiasmal lesions in the optic tract but before the lateral geniculate nucleus produce contralateral homonymous hemianopsia, contralateral RAPD, as well as contralateral band type (bow-tie) optic atrophy.
Post-geniculate body lesions in adults involving the optic radiations or the occipital cortex do not produce loss of visual acuity, an RAPD, or optic atrophy. PVL and other in utero or neonatal insults, however, can produce trans-synaptic degeneration across the lateral geniculate body and thus produce optic atrophy mimicking pre-geniculate lesions in adults. PVL involvement of extrastriate association cortex may result in other classical findings of difficulties with object recognition, motion detection, and visual attention10. Although post-geniculate lesions in the fully developed adult brain do not usually produce optic atrophy, ophthalmologists caring for adult patients with a history of PVL need to be aware that in utero cortical events can cause transsynaptic degeneration and secondary optic atrophy, band atrophy, or pseudo-glaucomatous cupping.
Primary prevention
PVL is anticipated to become more prevalent due to advances in perinatal care and consequent improved survivorship. Prenatal treatment focusing on delaying premature membrane rupture and parturition would prevent PVL by allowing more time for periventricular vascular development and by avoiding hypoxic-ischemic events. In cases where perinatal hypoxic brain injury has already occurred, use of hypothermia as neuroprotective treatment has been studied16.
Diagnosis
Symptoms & Signs
The severity and extent of the ophthalmic ocular manifestations of PVL are typically dependent on the degree of cerebral injury. Significant visual involvement classically presents in infants and children with symptoms of visual inattention, diplopia, difficulty with fixating on faces, perception of motion, depth perception4, and difficulty maintaining eye contact12. Esotropia and nystagmus may also be present11,13. These ophthalmic manifestations are well known to pediatric ophthalmologists.
For ophthalmologists caring for adult patients with a history of childhood PVL, it is essential to understand the nuances that differentiate PVL related pseudo-glaucomatous cupping from normal tension glaucoma. Patients with PVL may be misdiagnosed with normal tension glaucoma and receive inappropriate treatment because of similar features of nerve cupping and visual field deficits7.
The associated emotional and financial burden associated with misdiagnosis with glaucoma can significantly impact quality of life of patients. Therefore, it is essential to raise awareness of PVL as a possible cause of strabismus, visual deficit and cupping in patients with history of prematurity and perinatal complication. Ascertaining patient history in patients with these fundoscopic and visual field findings should be the first step in driving towards suspicion and diagnosis of PVL.
Physical examination
Ocular examination of adult patients with history of prematurity includes a full neuro-ophthalmic exam including formal, automated perimetry, color vision testing, pupillary exam, and dilated fundus examination. The differentiating features on examination of pre-chiasmal versus post chiasmal and pre-geniculate versus post-geniculate body visual loss are described in Table 1.
| Unilateral pre-chiasmal lesion | Post-chiasmal, pre-geniculate lesion | Unilateral post-chiasmal, post-geniculate lesion |
| Ipsilateral visual acuity or visual field loss | Contralateral homonymous hemianopsia | Contralateral homonymous hemianopsia,
No loss of visual acuity |
| Optic nerve atrophy | Contralateral band type optic atrophy | No optic nerve atrophy |
| Dyschromatopsia | No dyschromatopsia | No dyschromatopsia |
| Ipsilateral relative afferent pupillary defect (RAPD) | Contralateral RAPD | No RAPD |
Table 1: Comparison of characteristic clinical features of anterior and posterior lesions of visual pathway. Note that perinatal postgeniculate injury such as PVL may produce optic atrophy via trans-synaptic degeneration.
The differentiating features of true glaucoma in adulthood versus pseudoglaucomatous cupping from PVL are described in Table 2.
| Glaucoma | PVL |
| Vertical cupping in eye with nasal visual field loss | Horizontal band cupping in eye with temporal visual field loss |
| Variable nerve fiber layer type visual field defects (often nasal step) | More prominent Inferior visual field defect (may be temporal) |
Table 2: Comparison of characteristic clinical features of normal tension glaucoma and PVL.
Diagnostic procedures & laboratory tests
Table 3 lists the findings on OCT that help to differentiate glaucomatous from pseudo-glaucomatous cupping in PVL.
| Glaucoma | Pseudoglaucomatous PVL |
| Hourglass type (superior and inferior retinal nerve fiber layer loss first). Sparing of papillomacular bundle (until late) | Diffuse or nasal thinning
May show thinning of papillomacular bundle |
| Progressive over time | Non-progressive and stable over time |
Table 3: Comparison of characteristic OCT findings of normal tension glaucoma and PVL.
Literature reviews revealed that MRI plays an important role in screening and diagnosing intracranial brain changes in children about visual function outcome. 18
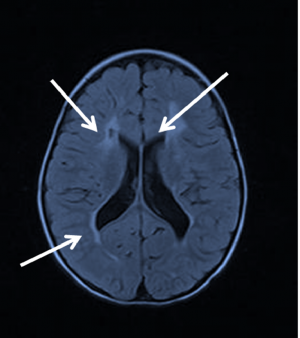
The characteristic neuroimaging features on computed tomography (CT) and magnetic resonance imaging (MRI) of PVL are summarized in Table 4. Increased signal intensity in the periventricular region on T2-weighted MRI and findings of decreased white matter in the periventricular region are diagnostic of PVL (Figure 1).
| CT | MRI |
| Hypodensity in periventricular white matter | Increased periventricular signal intensity w/ T2 MRI |
| Deep, prominent sulci w/ ventriculomegaly | White matter volume loss in T1 MRI |
| Irregular lateral ventricle outline |
Table 4: Characteristic features of PVL on CT and on MRI. Findings are usually consistent with white matter loss and thinning of periventricular region. The topographical anatomy of the PVL injury typically correlates with the the type and severity of the visual field defect. The early signs of PVL include periventricular white matter necrosis followed by the subacute stage with possible intraparenchymal cyst formation and the late stages of parenchymal loss and enlargement of the ventricles.
The extent of PVL may be further assessed using fMRI with various visual stimuli. In cases where assessment of visual acuity is difficult, flash visual evoked potentials have been used to estimate visual acuity14,15. Laboratory testing is not typically necessary for PVL diagnosis.
Differential diagnosis
As has been discussed, this condition is often mistaken for normal tension glaucoma. However, other differential diagnoses include ischemic, infectious, inflammatory, compressive, congenital, and toxic-nutritional etiologies.
Management
Medical therapy
The treatment of PVL in general should be directed at the underlying etiology. Optimal management of PVL includes not only care for ocular complaints but also interdisciplinary management involving speech therapy, physiotherapy, and cognitive therapy.
Medical follow up
Longitudinal follow-up with repeat visual field and OCT are helpful in differentiating PVL related optic atrophy from normal tension glaucoma. Adjustment for refractive error may periodically be needed, as patients with PVL may commonly present with astigmatism or hyperopia17.
Surgery
Surgical intervention is typically not warranted in PVL.
Complications
Since PVL is typically the result of perinatal hypoxic injury, progression and further complications are not common.
Prognosis
Visual impairment with PVL may improve with time. However, extent of improvement, if any, is highly dependent on degree of cerebral injury as well as time of diagnosis and of starting rehabilitation.
References
1. Levene MI, Wigglesworth JS, Dubowitz V. Hemorrhagic periventricular leukomalacia in the neonate: a real-time ultrasound study. Pediatrics. 1983;71(5):794-797.
2. Leech R, Alford E. Morphologic variations in periventricular leukomalacia. Am J Pathol. Published online 1974. doi:74:591-600
3. Schellinger D, Grant EG, Richardson JD. Cystic periventricular leukomalacia: sonographic and CT findings. Am J Neuroradiol. 1984;5(4):439-445.
4. Jacobson LK, Dutton GN. Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Surv Ophthalmol. 2000;45(1):1-13. doi:10.1016/s0039-6257(00)00134-x
5. Zaghloul. Pathophysiology of periventricular leukomalacia: What we learned from animal models. Accessed November 30, 2021. https://www.nrronline.org/article.asp?issn=1673-5374;year=2017;volume=12;issue=11;spage=1795;epage=1796;aulast=Zaghloul
6. Khurana R, Shyamsundar K, Taank P, Singh A. Periventricular leukomalacia: an ophthalmic perspective. Med J Armed Forces India. 2021;77(2):147-153. doi:10.1016/j.mjafi.2020.05.013
7. Groth SL, Donahue SP, Reddy A, Sarma A, Wushensky C. Periventricular Leukomalacia in Patients With Pseudo-glaucomatous Cupping. Am J Ophthalmol. 2020;211:31-41. doi:10.1016/j.ajo.2019.10.016
8. Kinney HC. The Near-Term (Late Preterm) Human Brain and Risk for Periventricular Leukomalacia: A Review. Semin Perinatol. 2006;30(2):81-88. doi:10.1053/j.semperi.2006.02.006
9. Melhem ER, Hoon AH, Ferrucci JT, et al. Periventricular leukomalacia: Relationship between lateral ventricular volume on brain MR images and severity of cognitive and motor impairment. RADIOLOGY. 2000;214(1):199-204. doi:10.1148/radiology.214.1.r00dc35199
10. Liu GT, Volpe NJ, Galetta SL. 3 - Visual Loss: Overview, Visual Field Testing, and Topical Diagnosis. In: Liu GT, Volpe NJ, Galetta SL, eds. Liu, Volpe, and Galetta’s Neuro-Ophthalmology (Third Edition). Elsevier; 2019:39-52. doi:10.1016/B978-0-323-34044-1.00003-1
11. Huo R, Burden SK, Hoyt CS, Good WV. Chronic cortical visual impairment in children: aetiology, prognosis, and associated neurological deficits. Br J Ophthalmol. 1999;83(6):670-675. doi:10.1136/bjo.83.6.670
12. Chhablani PP, Kekunnaya R. Neuro-ophthalmic manifestations of prematurity. Indian J Ophthalmol. 2014;62(10):992-995. doi:10.4103/0301-4738.145990
13. Volpe JJ. Neurobiology of Periventricular Leukomalacia in the Premature Infant. Pediatr Res. 2001;50(5):553-562. doi:10.1203/00006450-200111000-00003
14. Jethani J, Jethani M. Flash visual evoked potentials in patients with periventricular leucomalacia in children less than 1 year of age. Indian J Ophthalmol. 2013;61(11):634-635. doi:10.4103/0301-4738.123146
15. Kato T, Okumura A, Hayakawa F, Kuno K, Watanabe K. The evolutionary change of flash visual evoked potentials in preterm infants with periventricular leukomalacia. Clin Neurophysiol. 2005;116(3):690-695. doi:10.1016/j.clinph.2004.09.025
16. Kapetanakis A, Azzopardi D, Wyatt J, Robertson NJ. Therapeutic hypothermia for neonatal encephalopathy: a UK survey of opinion, practice and neuro-investigation at the end of 2007. Acta Paediatr. 2009;98(4):631-635. doi:10.1111/j.1651-2227.2008.01159.x
17. Ganesh S, Khurana R, Wallang B, Sharma S. Ophthalmic Manifestations in Children with Periventricular Leukomalacia. Indian J Pediatr. 2018;85(7):572-572. doi:10.1007/s12098-018-2643-y
18. Stefania Petri 1, Francesca Tinelli 2.Visual impairment and periventricular leukomalacia in children: A systematic review. Red Dev Disabil. 2023 Apr:135:104439. doi: 10.1016/j.ridd.2023.104439. Epub 2023 Feb 14.PMID: 36796269