Neuro-Ophthalmic Findings in Krabbe Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Krabbe disease, or globoid cell leukodystrophy, is an autosomal recessive lysosomal storage disease characterized by progressive neurodegeneration due to a genetic galactocerebrosidase-beta galactosidase (galactosylceramidase) deficiency.[1] It typically presents with bilateral loss of visual acuity, supine postural nystagmus, and extraocular movement defects.[1]
Disease
There are infantile, juvenile, and adult-onset forms of Krabbe disease, with the infantile-onset form (onset <12 months) being most common.[2]
The infantile form typically develops around 6 months of age, presenting with irritability and delayed motor development. Most patients succumb to the illness between 2 and 4 years of age.[3][4]
The later onset variant features slower progression than the adult form, which typically progresses over more than 10 years. [3][4]
Etiology
Krabbe disease is a rare autosomal recessive disorder.[1] Mutations in the β-galactocerebrosidase gene on chromosome 14 result in decreased or absent functioning levels of the enzyme galactosylceramide beta hydrolase (GALC).[5] Reduction of GALC activity causes an accumulation of galactocerebroside and psychosine, which are highly cytotoxic lipids.[6] Accumulation of galactocerebroside can be found in many visceral organs of affected individuals. It is especially toxic to oligodendrocytes and myelin, causing rapid and fatal neurodegeneration.[7]
The galc gene is a 60kb gene in length, containing 17 exons and 16 introns.[8] Over 130 distinct mutations, resulting from missense, nonsense, deletions, and insertions, have been cataloged in the Human Gene Mutation Database (HGMD).[9]
Of note, individuals with homozygous mutations of the GALC gene tend to demonstrate earlier disease progression, and overall mortality rates for infancy-onset Krabbe disease is >90%.[10][11] Genetic research also indicates that the infantile form of Krabbe disease often has mutations in the central domain, while the adult-onset form results from mutations in the N-terminus or C-terminus.[12]
Additionally, large kilobase (30kb) deletions to the GALC gene are the most common mutation and correlate with severe manifestations.[8] In Northern Europe, 30kb deletions are responsible for 40%-45% of the mutations in the infantile form. A similar trend was observed in Mexico with 30kb deletions being present in 35% of infantile form patients.[13]
Vision loss is a common manifestation of Krabbe disease. Accumulation of galactocerebroside and psychosine causes retrograde degeneration of the optic nerve, ultimately leading to loss of retinal axons and ganglion cells.[14]
Pathophysiology
Krabbe disease is caused by a deficiency of galactocerebrosidase. This enzyme functions as an acid hydrolase that degrades galactocerebrosides and sphingolipids, both of which are components of myelin. Their disruption eventually leads to the toxic accumulation of derivative byproducts in oligodendrocytes and Schwann cells.[2]
Despite the identification of over 75 pathogenic GALC mutations, a mutation that predicts age of onset remains unknown.[3]
Risk Factors
Due to the autosomal recessive nature of inheritance, key risk factors for Krabbe disease include:
Diagnosis
History
As Krabbe disease must be diagnosed early for currently available therapies to slow progression, a thorough history should be taken when suspecting Krabbe or other lysosomal storage diseases, including:
- Age of onset[15]
- Delayed or missed developmental milestones[15]
- Family history of neurodevelopmental disorders[15]
- Change in behavior/mood[15]
Physical examination
A thorough ophthalmic and neurologic exam should be performed. Positive neuro-ophthalmic findings can include:
- Bilateral loss of visual acuity to total blindness[4]
- Supine postural nystagmus[4]
- Poor optokinetic responses[4]
- Symmetric CN III and CN VI palsies[4]
Positive neurologic findings can include:
- Spastic paraparesis[4][16]
- Opisthotonus[4][16]
- Decerebrate rigidity[4][16]
- Dysarthria[4][16]
- Cerebellar ataxia[4][16]
- Tongue atrophy[4][16]
- Cognitive decline[4][16]
Ocular Findings
Slit lamp and dilated fundoscopic examination can be used to rule out anterior segment pathology along with other commonly identifiable etiologies. Both are typically unremarkable in Krabbe disease.[17] Posterior pole findings that have been reported include subtle cherry red spots and optic atrophy. [18]
Laboratory Testing & Imaging
The guidelines regarding laboratory testing vary based on the age of onset. Newborn screening for Krabbe disease is performed in a growing minority of states in the US. It is performed using tandem mass spectrometry assays followed by a second confirmatory tier of testing typically including full Sanger sequencing of the GALC gene in highly suspected cases.[15][16]
Brain MRI can be used to identify the location of demyelinating lesions, with white matter hyperintensities commonly affecting heterogenous locations along the corticospinal tract, the corpus callosum, and the optic radiations.[4][19] Enhancement of cerebral lesions is generally rare, but enhancement of cranial nerves have also been reported.[20] Interestingly, optic nerve enlargement is an uncommon finding that contradicts the diffuse white matter atrophy seen in Krabbe disease.[17]
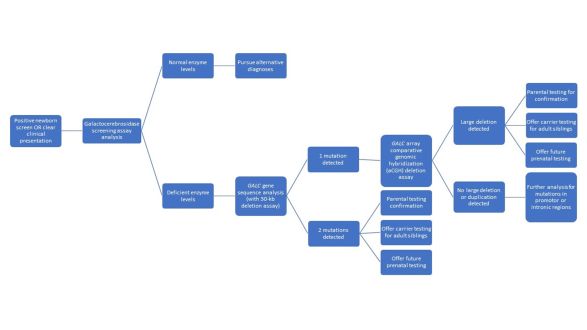
A diagnostic algorithm summarizing the steps clinicians should take when evaluating patients for Krabbe disease is shown below.
Differential diagnosis
Differential Diagnosis
- Metachromatic leukodystrophy[21][22]
- GM1/GM2-gangliosidosis (and other Hexosaminidase A deficiency disorders)[22][23]
- X-linked adrenoleukodystrophy[22]
- Pelizaeus-Merzbacher disease[22]
- Canavan disease[22][24]
- Alexander disease[22][25]
Management & Outcomes
Currently, there are no curative treatments for Krabbe disease. Most management options focus on symptomatic treatment and supportive care.[3] Of note, hematopoietic stem cell transplantation (HSCT) is a disease-modifying treatment that can slow the progression of infantile-onset Krabbe disease.[26] However, HSCT is only effective in delaying progression of infantile-onset Krabbe disease if initiated before symptomatic onset.[2] There are no therapies available for adult-onset Krabbe disease.
Routine newborn screening for Krabbe disease is not currently recommended due to its severely limited treatment options and low prevalence.[27] Individuals with confirmed Krabbe disease should have an eye examination and otolaryngology assessment.
Exploration of combination therapies that include HSCT, enzyme replacement therapy (ERT) of β-galactocerebrosidase, and substrate reduction therapy (SRT) of galactocerebroside and psychosine and have yielded varying results in twitcher mice models.[28] Translation of these studies to human models have been severely limited by the interaction of these targeted enzymes and substrates with numerous other physiologic processes that share similar metabolic pathways.[2]
Very recently, small trials on adeno-associated virus (AAV) gene therapies in twitcher mice, rhesus monkey, and canine studies have yielded encouraging results, demonstrating significant improvements in mortality, symptoms, and disease progression for these animal models.[29][30][31]
Krabbe disease is a rapidly-progressing disorder, with most patients succumbing before the age of 2.[3] Despite the poor prognosis of Krabbe disease, advancing understanding of its pathophysiology and the advent of new treatments for complex genetic diseases, provide promise for improvements both management and prognosis.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Graziano ACE, Cardile V. History, genetic, and recent advances on Krabbe disease. Gene. 2015;555(1):2-13. doi:10.1016/j.gene.2014.09.046
- ↑ 2.0 2.1 2.2 2.3 Bradbury AM, Bongarzone ER, Sands MS. Krabbe disease: New hope for an old disease. Neurosci Lett. 2021;752:135841. doi:10.1016/j.neulet.2021.135841
- ↑ 3.0 3.1 3.2 3.3 3.4 Escolar ML, West T, Dallavecchia A, Poe MD, LaPoint K. Clinical management of Krabbe disease. J Neurosci Res. 2016;94(11):1118-1125. doi:10.1002/jnr.23891
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 Debs, R., Froissart, R., Aubourg, P., Papeix, C., Douillard, C., Degos, B., Fontaine, B., Audoin, B., Lacour, A., Said, G., Vanier, M. T., & Sedel, F. (2013). Krabbe disease in adults: Phenotypic and genotypic update from a series of 11 cases and a review. Journal of Inherited Metabolic Disease, 36(5), 859–868. https://doi.org/10.1007/s10545-012-9560-4
- ↑ Suzuki K, Suzuki Y. Globoid Cell Leucodystrophy (Krabbe’s Disease): Deficiency of Galactocerebroside β-Galactosidase*. Proc Natl Acad Sci. 1970;66(2):302-309. doi:10.1073/pnas.66.2.302
- ↑ Tanaka K, Nagara H, Kobayashi T, Goto I. The twitcher mouse: accumulation of galactosylsphingosine and pathology of the sciatic nerve. Brain Res. 1988;454(1-2):340-346. doi:10.1016/0006-8993(88)90835-9
- ↑ Suzuki K, Suzuki Y, Fletcher TF. Further Studies on Galactocerebroside β-Galactosidase in Globoid Cell Leukodystrophy. In: Volk BW, Aronson SM, eds. Sphingolipids, Sphingolipidoses and Allied Disorders: Proceedings of the Symposium on Sphingolipidoses and Allied Disorders Held in Brooklyn, New York, October 25–27, 1971. Advances in Experimental Medicine and Biology. Springer US; 1972:487-498. doi:10.1007/978-1-4757-6570-0_33
- ↑ 8.0 8.1 Luzi P, Rafi MA, Wenger DA. Structure and organization of the human galactocerebrosidase (GALC) gene. Genomics. 1995;26(2):407-409. doi:10.1016/0888-7543(95)80230-J
- ↑ Wenger DA. Murine, canine and non-human primate models of Krabbe disease. Mol Med Today. 2000;6(11):449-451. doi:10.1016/S1357-4310(00)01800-1
- ↑ Furuya H, Kukita Y ji, Nagano S, et al. Adult onset globoid cell leukodystrophy (Krabbe disease): analysis of galactosylceramidase cDNA from four Japanese patients. Hum Genet. 1997;100(3-4):450-456. doi:10.1007/s004390050532
- ↑ Jain M, De Jesus O. Krabbe Disease. In: StatPearls. StatPearls Publishing; 2022. Accessed July 17, 2022. http://www.ncbi.nlm.nih.gov/books/NBK562315/
- ↑ De Gasperi R, Gama Sosa MA, Sartorato EL, et al. Molecular heterogeneity of late-onset forms of globoid-cell leukodystrophy. Am J Hum Genet. 1996;59(6):1233-1242.
- ↑ Rafi MA, Luzi P, Chen YQ, Wenger DA. A large deletion together with a point mutation in the GALC gene is a common mutant allele in patients with infantile Krabbe disease. Hum Mol Genet. 1995;4(8):1285-1289. doi:10.1093/hmg/4.8.1285
- ↑ Brownstein S. Optic Nerve in Globoid Leukodystrophy (Krabbe’s Disease): Ultrastructural Changes. Arch Ophthalmol. 1978;96(5):864. doi:10.1001/archopht.1978.03910050466015
- ↑ 15.0 15.1 15.2 15.3 15.4 Kwon, J. M., Matern, D., Kurtzberg, J., Wrabetz, L., Gelb, M. H., Wenger, D. A., Ficicioglu, C., Waldman, A. T., Burton, B. K., Hopkins, P. V., & Orsini, J. J. (2018). Consensus guidelines for newborn screening, diagnosis and treatment of infantile Krabbe disease. Orphanet Journal of Rare Diseases, 13(1), 30. https://doi.org/10.1186/s13023-018-0766-x
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 Shekhar, S., Natteru, P., Khan, M. A., Desai, J., & Patel, R. (2018). Diffuse Cranial Nerve Involvement in a Patient With Early Infantile Krabbe Disease. Pediatric Neurology, 87, 75–76. https://doi.org/10.1016/j.pediatrneurol.2018.07.009
- ↑ 17.0 17.1 Castilha-Neto, D., Fernandes Monteiro, L., Maccarini Peruchi, M., Moreno Filho, J., Scarlatelli-Lima, A. V., & Lin, J. (2012). Optic nerve enlargement in infantile form of Krabbe disease. Clinics and Practice, 2(4), e81.
- ↑ Naidu S, Hofmann KJ, Moser HW, Maumenee IH, Wenger DA. Galactosylceramide-beta-galactosidase deficiency in association with cherry red spot. Neuropediatrics. 1988 Feb;19(1):46-8. doi: 10.1055/s-2008-1052400. PMID: 3362311.
- ↑ Cousyn, L., Law-Ye, B., Pyatigorskaya, N., Debs, R., Froissart, R., Piraud, M., Federico, A., Salvatore, S., Cerase, A., Macário, M. C., Durães, J., Kim, S. H., Adachi, H., Audoin, B., Ayrignac, X., Da, Y., Henderson, R., La Piana, R., Laule, C., … Nadjar, Y. (2019). Brain MRI features and scoring of leukodystrophy in adult-onset Krabbe disease. Neurology, 93(7), e647–e652. https://doi.org/10.1212/WNL.0000000000007943
- ↑ Ganesan K, Desai S, Hegde A. Multiple cranial nerve enhancement: uncommon imaging finding in early infantile Krabbe's disease. J Neuroimaging. 2010 Apr;20(2):195-7. doi: 10.1111/j.1552-6569.2008.00308.x. Epub 2008 Oct 21. PMID: 19021834.
- ↑ Kohlschütter A. Lysosomal leukodystrophies: Krabbe disease and metachromatic leukodystrophy. Handb Clin Neurol. 2013;113:1611-1618. doi:10.1016/B978-0-444-59565-2.00029-0
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 Orsini JJ, Escolar ML, Wasserstein MP, Caggana M. Krabbe Disease. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews®. University of Washington, Seattle; 1993. Accessed July 17, 2022. http://www.ncbi.nlm.nih.gov/books/NBK1238/
- ↑ Wajner A, Michelin K, Burin MG, et al. Comparison between the biochemical properties of plasma chitotriosidase from normal individuals and from patients with Gaucher disease, GM1-gangliosidosis, Krabbe disease and heterozygotes for Gaucher disease. Clin Biochem. 2007;40(5):365-369. doi:10.1016/j.clinbiochem.2006.12.003
- ↑ Kwan E, Drace J, Enzmann D. Specific CT findings in Krabbe disease. Am J Roentgenol. 1984;143(3):665-670. doi:10.2214/ajr.143.3.665
- ↑ Kwan E, Drace J, Enzmann D. Specific CT findings in Krabbe disease. Am J Roentgenol. 1984;143(3):665-670. doi:10.2214/ajr.143.3.665
- ↑ Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of Umbilical-Cord Blood in Babies with Infantile Krabbe’s Disease. N Engl J Med. 2005;352(20):2069-2081. doi:10.1056/NEJMoa042604
- ↑ Kemper AR, Knapp AA, Green NS, Comeau AM, Metterville DR, Perrin JM. Weighing the evidence for newborn screening for early-infantile Krabbe disease. Genet Med. 2010;12(9):539-543. doi:10.1097/GIM.0b013e3181e85721
- ↑ Mikulka CR, Sands MS. Treatment for Krabbe’s disease: Finding the combination: Treatment for Krabbe’s Disease: Unlocking the Combination. J Neurosci Res. 2016;94(11):1126-1137. doi:10.1002/jnr.23822
- ↑ Bradbury AM, Bagel JH, Nguyen D, et al. Krabbe disease successfully treated via monotherapy of intrathecal gene therapy. J Clin Invest. 2020;130(9):4906-4920. doi:10.1172/JCI133953
- ↑ Isakova IA, Baker KC, Dufour J, Phinney DG. Mesenchymal Stem Cells Yield Transient Improvements in Motor Function in an Infant Rhesus Macaque with Severe Early-Onset Krabbe Disease. Stem Cells Transl Med. 2017;6(1):99-109. doi:10.5966/sctm.2015-0317
- ↑ Karumuthil-Melethil S, Marshall MS, Heindel C, Jakubauskas B, Bongarzone ER, Gray SJ. Intrathecal administration of AAV/GALC vectors in 10–11-day-old twitcher mice improves survival and is enhanced by bone marrow transplant. J Neurosci Res. 2016;94(11):1138-1151. doi:10.1002/jnr.23882