Mycoplasma-Induced Rash and Mucositis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Descriptive article of the ophthalmic manifestations of Mycoplasma-Induced Rash and Mucositis
Disease Entity
Mycoplasma-Induced Rash and Mucositis (MIRM) is recognized by the following codes as per the International
Classification of Diseases (ICD) nomenclature:
ICD 9
- ICD-9-CM
- 041.81 Other specified bacterial infections in conditions classified elsewhere and of unspecified site, mycoplasma
ICD 10
- ICD-10-CM
- B96.0 – Mycoplasma pneumonia as the cause of diseases elsewhere[1]
- B96.0 – Mycoplasma pneumonia as the cause of diseases elsewhere[1]
Disease
Mycoplasma-Induced Rash and Mucositis (MIRM), also known as Mycoplasma pneumoniae-associated mucositis (MPAM) is an extrapulmonary manifestation of Mycoplasma pneumoniae (M. pneumoniae) that has recently been distinguished from Erythema Multiforme (EM), Stevens-Johnson Syndrome (SJS), and Toxic Epidermal Necrolysis (TEN).[2][3][4][5]This disease is characterized by a severe mucositis that predominantly affects the oral mucosa, but can involve the conjunctiva and genital mucosa as well.[2] Ocular involvement is variable, but it generally includes a bilateral conjunctivitis without chemosis and notably, very rarely has corneal involvement. Other ophthalmic features can include mucoid discharge, conjunctival epithelial defects, eyelid margin hyperemia and staining, and/or pseudomembranes.[4] While there is limited data published regarding the ocular manifestations of MIRM, the current data suggests that MIRM typically has a more limited ocular course and a more favorable ocular prognosis compared to SJS/TEN.[4]
Definitions
Previously, M. pneumoniae associated mucocutaneous eruptions were labeled with different designations along the spectrum of erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) based on the varied presentations of these cases.[2][5][6][7][8] However, recent studies have separated MIRM as a distinct entity, including a large systematic review by Canavan et al.[2][3][4][5] Some distinguishing features include its predilection for young patients, predominant mucosal involvement, sparse vesiculobullous and/or targetoid cutaneous eruption, and great overall prognosis.[2] More distinguishing traits are summarized in Table 1 and in the respective sections below.
| Table 1: Distinguishing characteristics comparing Mycoplasma-induced rash and mucositis (MIRM), erythema multiforme (EM), and Stevens-Johnson syndrome (SJS) / Toxic Epidermal Necrolysis (TEN).[9] | |||
|---|---|---|---|
| MIRM | EM | SJS/TEN | |
| Trigger | M. Pneumonia | HSV | Drug induced |
| Patient Demographics | Younger males | Younger males | Adults |
| Rash Characteristics | Prominent mucositis, limited cutaneous vesiculobullous lesions +/- targetoid eruption | Classic acral target lesions | Extensive and central target lesions with potential for extensive detachment (TEN) |
| Frequency of Ocular Disease | 82% | 5-23% | 50-88% |
| Mortality | 3-4% | 0-6% | 25-30% |
Epidemiology
Mycoplasma pneumoniae (M. pneumoniae) is a common cause of skin and respiratory infections, pneumonitis, bronchitis, and febrile upper respiratory infections.[10] M. pneumoniae is also an important cause of community-acquired pneumonia in children, which often has extra-pulmonary manifestations in up to 25% of patients, most commonly mucocutaneous involvement as in MIRM.[11][12] MIRM typically affects younger patients and has less severe cutaneous findings and a more favorable prognosis compared to SJS/TEN.[2][3] MIRM commonly involves more than one mucous membrane, most prominently the oral mucosa with vesiculobullous lesions and extensive oral erosions.[13] A systematic review of patients with MIRM found that most patients were young males (66%), with a mean age of 11.9 years old, and ocular findings were present in 82% of these patients.[2]
Pathophysiology
The precise pathophysiology of MIRM is not completely understood. One proposed mechanism is polyclonal B-cell proliferation and antibody production with subsequent immune complex deposition and complement activation that could cause skin damage. Another possibility is molecular mimicry between Mycoplasma P1-adhesion molecules and a host's keratinocytes.[2] Conversely, erythema multiforme type diseases likely involve cytotoxicity mediated through a perforin/granzyme pathway with Fas ligand and granulysin and a type IV delayed-type hypersensitivity reaction.[2]
History
MIRM typically affects younger patients with a slight male predominance. Patients generally have a prodrome of fever, malaise, and cough approximately one week prior to the onset of mucocutaneous eruption.[2][4] Mortality is low, most patients have a full recovery from their disease, and recurrence is uncommon (8%).[2] Prominent involvement of the mucosal membranes with limited cutaneous involvement is the common presentation; however, there are forms with no cutaneous involvement, which can be classified as MIRM sine rash .[2][14][15]
Diagnosis
MIRM is a diagnosis made by a combination of clinical, radiographic, and laboratory findings, and does require a high level of suspicion.
Physical Examination
Most patients are seen in the inpatient setting; therefore, the exam can be performed with an indirect ophthalmoscope, 20 diopter lens, fluorescein stain, and cobalt blue light source to evaluate the anterior segment. A thorough evaluation of the cornea, conjunctiva (bulbar and palpebral), and eyelids (including mucocutaneous junction) is necessary to evaluate the extent of ocular involvement. One must assess for the presence of epithelial sloughing as evidenced by fluorescein staining on the above surfaces. In addition, a combined effort with dermatology and urology is useful for evaluation of dermatologic manifestations and for evaluation/treatment of patients with extensive genital mucosal involvement.
Signs
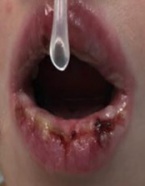
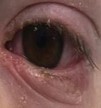
MIRM features a prominent mucositis and almost always has oral involvement that can be in the form of vesiculobullous lesions and extensive erosions (Figure 1).[2][13] It typically involves 2.5 mucosal sites, most commonly ocular and oral, and a characteristic cutaneous sparse vesiculobullous and/or targetoid eruption.[2] Ocular involvement is varied but generally includes a bilateral conjunctivitis without chemosis or corneal involvement. Additional potential findings include mucoid discharge, conjunctival epithelial defects, eyelid margin hyperemia and staining, and/or pseudomembranes (Figure 2).[4][16] These ocular findings may initially be subtle, and thus the need for close ophthalmology follow up both inpatient and outpatient are important to prevent permanent ocular surface damage from MIRM.
Symptoms
Ocular symptoms are not as well described in the current literature, but can include redness, tearing, photophobia, and blurry vision.[2][4]
Clinical Diagnosis
The diagnosis of MIRM depends on the typical features as described above and evidence of recent infection with M. Pneumoniae. Diagnostic criteria for typical cases of MIRM proposed by Canavan et. al are summarized below. These criteria also apply to atypical cases but with different skin findings. MIRM sine rash has few fleeting morbilliform lesions or few vesicles, while severe MIRM has extensive widespread blisters or flat atypical targets.[2]
Typical MIRM Criteria:
- <10% BSA with detachment:
- > 2 mucosal sites involved
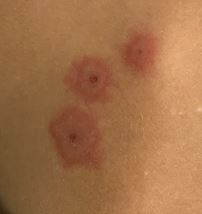
- Few cutaneous vesiculobullous lesions, or scattered atypical targets
- Presence or absence of targetoid lesions (Figure 3)
- Atypical pneumonia
- Signs: fever, cough, auscultatory or radiographic findings
- Labs: increase in M. Pneumoniae IgM antibodies, M. Pneumoniae in oropharyngeal or bullae cultures or PCR, and/or serial cold agglutinins
Laboratory test
Laboratory testing for M. Pneumoniae includes: isolation, complement fixation, molecular-based detection assays such as PCR, and serologic testing.[17] Serologic testing demonstrating IgM antibodies for M. Pneumoniae suggests recent infection, but these levels can remain elevated for several months. Therefore, more accurate diagnosis is found with paired serologic testing that demonstrates either seroconversion from absence of IgM to presence of IgM or four-fold increase of IgG titers from acute to convalescent phase samples.[18]
Differential diagnosis
Since MIRM predominantly affects the pediatric population the differential diagnosis summarized in Table 2 lists possibilities more prevalent in this demographic.
| Table 2. Differential Diagnosis |
|---|
| Kawasaki Disease |
| Erythema Multiforme |
| Stevens-Johnson Syndrome |
| Toxic Epidermal Necrolysis |
| Varicella Zoster |
| Viral Exanthem |
Prognosis
MIRM has a better overall prognosis compared to SJS and TEN with regards to morbidity and mortality.[2][3][4][9] Ocular complications of EM and SJS/TEN are well described in the literature and can result in debilitating sequelae, including symblepharon, keratinized mucosal surfaces, scarred lid margins, entropion, trichiasis, limbal stem cell failure, and corneal scarring.[19][20][21][22] Based on the systematic review by Canavan et al. ophthalmic consequences of MIRM can include corneal ulcers, conjunctival shrinkage, blindness, synechiae, dry eyes and madarosis; however, these were present in only 8.9% of cases.[2] One more recent case series with far fewer patients demonstrated that with proper topical treatment most patients have a complete ocular recovery with BCVA 20/25 or better and only one patient suffered from eyelid margin scarring.[4] Another case series of 15 patients noted on follow up no vision loss but 1 patient with mild symblephara and 2 patients with residual eyelid margin scarring. [23] Few cases of corneal epithelial defects and periorbital edema have been reported.[24]
Management
General treatment
Currently there is no standardized treatment regimen for patients with MIRM; however, the treatment guidelines for SJS by Gregory can be applied to these patients given the similar presentation, and systemic treatment for the underlying Mycoplasma pneumoniae is critical. The Gregory guidelines for treatment of SJS categorize patients into mild, moderate, severe, and extremely severe based on the location and extent of conjunctival and corneal epithelial sloughing. This epithelial involvement can be measured with the amount of fluorescein staining. Patients that fall in the mild and moderate categories were treated medically with a combination of drops and those that were severe or extremely severe required surgical amniotic membrane transplant (AMT) in addition to medical therapy.[20] The criteria that stratified patients as severe was involvement of more than 1/3 of the eyelid margin, and/or more than 10 cm in diameter of bulbar conjunctiva, with or without more than punctate staining of the cornea.[20] In one case series of 15 patients, 3 patients required AMT and 1 a sutureless biologic corneal bandage device while the remainder were medically managed.[23]
Medical therapy
Topical therapy following the SJS treatment protocol consists of an antibiotic drop QID, cyclosporine 0.05% drops BID, steroid drops BID, and an antibiotic or combination antibiotic/steroid ointment to eyelid margins BID to QID.[20]
Surgery
Surgical therapy is primarily AMT once patients meet criteria as listed above. Importantly AMT should be performed within seven to 10 days from onset of epithelial sloughing as prior studies have shown decreased efficacy past this time frame.[19][20][25]
Additional Recommendations
Patients with MIRM may have less impressive initial findings; however, like SJS/TEN these patients have a dynamic disease course that can vary drastically on a daily basis. Left untreated, patients are still at risk, albeit lower, of symblepharon and ocular surface scarring. Therefore, close follow-up with daily ophthalmic exam while patients are hospitalized is crucial to promptly institute necessary medical and/or surgical therapy in order to prevent permanent sequelae.
References
- ↑ # ICD10Data. Accessed April 10, 020. https://www.icd10data.com/
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72(2):239-245. doi:10.1016/j.jaad.2014.06.026
- ↑ 3.0 3.1 3.2 3.3 Prindaville B, Newell BD, Nopper AJ, Horii KA. Mycoplasma pneumonia--associated mucocutaneous disease in children: dilemmas in classification. Pediatr Dermatol. 2014;31(6):670-675. doi:10.1111/pde.12482
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Shah PR, Williams AM, Pihlblad MS, Nischal KK. Ophthalmic Manifestations of Mycoplasma-Induced Rash and Mucositis. Cornea. 2019;38(10):1305-1308. doi:10.1097/ICO.0000000000001985
- ↑ 5.0 5.1 5.2 Martínez-Pérez M, Imbernón-Moya A, Lobato-Berezo A, Churruca-Grijelmo M. Mycoplasma pneumoniae-Induced Mucocutaneous Rash: A New Syndrome Distinct from Erythema Multiforme? Report of a New Case and Review of the Literature. Actas Dermosifiliogr. 2016;107(7):e47-51. doi:10.1016/j.ad.2015.09.023
- ↑ Forman R, Koren G, Shear NH. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a review of 10 years’ experience. Drug Saf. 2002;25(13):965-972. doi:10.2165/00002018-200225130-00006
- ↑ Roujeau JC. Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol. 1997;24(11):726-729. doi:10.1111/j.1346-8138.1997.tb02524.x
- ↑ Tay YK, Huff JC, Weston WL. Mycoplasma pneumoniae infection is associated with Stevens-Johnson syndrome, not erythema multiforme (von Hebra). J Am Acad Dermatol. 1996;35(5 Pt 1):757-760. doi:10.1016/s0190-9622(96)90732-x
- ↑ 9.0 9.1 Chang Y-S, Huang F-C, Tseng S-H, Hsu C-K, Ho C-L, Sheu H-M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea. 2007;26(2):123-129. doi:10.1097/ICO.0b013e31802eb264
- ↑ Grayston JT, Alexander ER, Kenny GE, Clarke ER,Fremont JC, Maccoll WA. MYCOPLASMA PNEUMONIAE INFECTIONS. CLINICAL AND EPIDEMIOLOGIC STUDIES. JAMA. 1965;191:369-374.doi:10.1001/jama.1965.03080050015004
- ↑ He X, Wang X, Zhang R, et al. Investigation of Mycoplasma pneumoniae infection in pediatric population from 12,025 cases with respiratory infection. Diagn Microbiol Infect Dis. 2013;75(1):22-27. doi:10.1016/j.diagmicrobio.2012.08.027
- ↑ Moreau JF, Watson RS, Hartman ME, Linde-Zwirble WT, Ferris LK. Epidemiology of ophthalmologic disease associated with erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis in hospitalized children in the United States. Pediatr Dermatol. 2014;31(2):163-168. doi:10.1111/pde.12158
- ↑ 13.0 13.1 Morand M, Hatami A. Multiple superficial oral mucoceles after Mycoplasma-induced mucositis. Pediatr Dermatol. 2018;35(4):e210-e211. doi:10.1111/pde.13515
- ↑ Poddighe D, Bruni P. Mycoplasma pneumoniae-induced rash and mucositis (MIRM): an unusual mild skin rash associated with severe mucosal involvement. BMJ Case Rep. 2017;2017. doi:10.1136/bcr-2017-220749
- ↑ Bowling M, Schmutzler T, Glick S. Mycoplasma pneumoniae-induced mucositis without rash in an 11-year-old boy. Clin Case Rep. 2018;6(3):551-552. doi:10.1002/ccr3.1400
- ↑ Khalili A, Ackerman IM, Gorski MG, Kodsi SR, Finelt N, Hymowitz MB. Ophthalmic findings of Mycoplasma-induced rash and mucositis (MIRM) distinct from Stevens-Johnson syndrome. J AAPOS. 2021;25(6):348.e1-348.e6. doi:10.1016/j.jaapos.2021.06.003
- ↑ Thurman KA, Walter ND, Schwartz SB, et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;48(9):1244-1249. doi:10.1086/597775
- ↑ Lee W-J, Huang E-Y, Tsai C-M, et al. Role of Serum Mycoplasma pneumoniae IgA, IgM, and IgG in the Diagnosis of Mycoplasma pneumoniae-Related Pneumonia in School-Age Children and Adolescents. Clin Vaccine Immunol CVI. 2017;24(1). doi:10.1128/CVI.00471-16
- ↑ 19.0 19.1 Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. Ophthalmology. 2011;118(5):908-914. doi:10.1016/j.ophtha.2011.01.046
- ↑ 20.0 20.1 20.2 20.3 20.4 Gregory DG. New Grading System and Treatment Guidelines for the Acute Ocular Manifestations of Stevens-Johnson Syndrome. Ophthalmology. 2016;123(8):1653-1658. doi:10.1016/j.ophtha.2016.04.041
- ↑ Kohanim S, Palioura S, Saeed HN, et al. Acute and Chronic Ophthalmic Involvement in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis - A Comprehensive Review and Guide to Therapy. II. Ophthalmic Disease. Ocul Surf. 2016;14(2):168-188. doi:10.1016/j.jtos.2016.02.001
- ↑ Sotozono C, Ang LPK, Koizumi N, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson syndrome. Ophthalmology. 2007;114(7):1294-1302. doi:10.1016/j.ophtha.2006.10.029
- ↑ 23.0 23.1 Gise R, Elhusseiny AM, Scelfo C, Mantagos IS. Mycoplasma Pneumoniae-Induced Rash and Mucositis: A Longitudinal Perspective and Proposed Management Criteria. Am J Ophthalmol. 2020;219:351-356. doi:10.1016/j.ajo.2020.06.010
- ↑ Haseeb A, Elhusseiny AM, ElSheikh RH, Tahboub MA, Kwan JT, Saeed HN. Ocular involvement in Mycoplasma induced rash and mucositis: A systematic review of the literature. Ocul Surf. 2023 Apr;28:1-10. doi: 10.1016/j.jtos.2022.11.007. Epub 2022 Nov 14. PMID: 36396020.
- ↑ Gregory DG. The ophthalmologic management of acute Stevens-Johnson syndrome. Ocul Surf. 2008;6(2):87-95. doi:10.1016/s1542-0124(12)70273-2