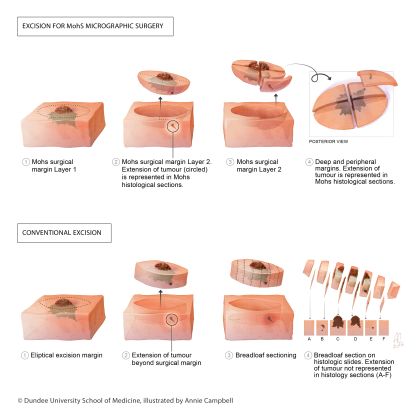
Mohs Micrographic Surgery
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Mohs micrographic surgery (MMS), also known as Mohs surgery, is a surgical technique for skin cancer removal.[1] It was conceptualized in the early 1930s by Dr. Frederic E. Mohs, who observed that cancer tissues treated with 20% zinc chloride solution maintained well-preserved microscopic morphology. This discovery set the foundation for a technique of removing cancer under microscopic control, the basis of Mohs surgery. The procedure was modified over the following decades; of note was the introduction of the fresh-tissue technique in 1953, which avoided zinc chloride fixation and worked particularly well on eyelid tumors.[2]
Multiple different surgical techniques exist for the treatment of periocular cutaneous malignancies, including wide local excision (WLE) and MMS. MMS utilizes frozen sections rather than permanent paraffin-embedded sections, thereby enabling same-day confirmation of clear margins prior to oculoplastic reconstruction. Further, Mohs surgery has been lauded for its efficacy and tissue-conserving nature, which is essential for periocular lesions given the importance of nearby highly-specialized tissues and the cosmetic implications of tumors and scars in this region. [1]
MMS is most frequently performed for basal cell carcinoma and squamous cell carcinoma.[3] However, it can offer utility in the removal of more rare periocular skin cancers as well, including melanoma. Historically, wide local excision was the primary approach for periocular melanomas, given the difficulty in discriminating between normal melanocytic hyperplasia of sun-damaged skin and true melanoma cells on frozen section. However, MMS with immunohistochemical (IHC) staining is an increasingly utilized option for thin melanomas and employs chromogen-tagged antibodies visible on light microscopy to detect antigens of interest, typically melanoma antigen recognized by T cells 1 (MART-1).[4] Recent studies have demonstrated high concordance rates (>96%) between MART-1 frozen sections and permanent sections for melanoma.[5][6][7]
Indications
Indications for Mohs surgery vary, but MMS is the standard of care for selected BCCs and SCCs and is commonly and increasingly used for melanomas and other tumors.
Clinicians are guided by the Appropriate Use Criteria (AUC) jointly developed by the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.[8] Briefly, the use of Mohs surgery is classified as "appropriate," "uncertain," or "inappropriate" based on individual tumor characteristics, anatomic location, and unique patient characteristics. The anatomic locations are divided into 3 areas: Area H (mask areas of face, genitalia, hands, feet, nail units, ankles, and nipples/areola), Area M (cheeks, forehead, scalp, neck, jawline, and pretibial surface), and Area L (trunk and extremities). There exists a free app, "Mohs Surgery Appropriate Use Criteria," which provides detailed guidance on whether to use MMS for the treatment of skin cancer in any given patient (available for download on Apple app store or Android PlayStore).
Surgical Technique
Mohs surgery is typically performed under local anesthesia, which is infiltrated into the tumor area and surrounding skin.
The first step of MMS is marking and excision of the clinically evident tumor (debulking). Then begins the first stage, when a small margin of normal tissue is removed with the blade oriented at a 45-degree angle to the skin, which allows for proper alignment of the peripheral edges of the tissue specimen during preparation of the specimen. Careful markings are made at the 12, 3, and 6 o'clock position to ensure orientation during histopathological analysis. Hemostasis is obtained and additional anesthetic may be administered while the patient awaits laboratory analysis of the initial specimen. The tissue is taken to the laboratory and flattened so the beveled peripheral margins are in the same plane as the deep margin, thereby ensuring circumferential histologic margin evaluation. A 2D map of the lesion and corresponding area is created using the same colors to identify each marking. The specimen is then frozen surrounded and fixed. The tissue is sectioned and multiple sections are placed on slides, stained with H&E and examined under the microscope by the Mohs surgeon. If skin cancer cells are detected, the process is repeated with expanded margins only around the area involved by residual cancer cells. This process is iterated until no skin cancer cells are detected under the microscope. Typically, clear margins are achieved after 1 or 2 stages and, in total, the procedure usually takes a few hours.[3][9]
Once margins are confirmed tumor-free, several avenues may be taken to manage the wound depending on its nature. It may be able to heal by secondary intent, or it may require primary closure or additional reconstructive techniques, such as grafts or flaps. This is frequently performed by the Mohs surgeon but may require multi-specialty collaboration if in anatomically complex or highly specialized areas, such as the eyelids. Often, the wound closure may be performed on the same day as the Mohs surgery.[3][10]
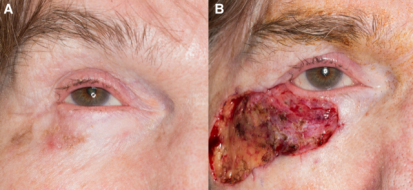
Advantages
The goal of Mohs surgery is to remove all histopathologic evidence of malignancy while conserving as much healthy skin as possible. The technique is particularly effective as it allows the practitioner to intraoperatively ensure microscopic evidence of tumor-free margins.[3] This is in contrast to wide local excision (WLE), in which specimens are typically processed in a cross-sectional or "bread-loaf" fashion, with representative tissue sliced vertically at 2- to 4- mm intervals to check for tumor cell presence. WLE thereby allows for evaluation of <1-2% of the entire surgical margin.
In addition to serving as an attestation of complete tumor excision, Mohs surgery has demonstrated advantages in sparing tissue and decreasing risk of recurrence in prior studies. One randomized trial reported a smaller median area of surgical defects in patients with basal cell carcinoma managed with Mohs surgery compared with standard surgery.[11] Another randomized trial documented a lower number of post-treatment recurrences in patients with high-risk facial basal cell carcinoma who were treated with Mohs surgery compared to those treated with traditional surgical excision.[12] Similar findings have been demonstrated for even melanoma of the head and neck, with MMS associated with improved local recurrence outcomes compared to wide-local excision, despite more frequent tumor location on high-risk anatomic sites, as well as favorable delay in time to local recurrence.[13]
Limitations
The limitations associated with Mohs surgery primarily concern tissue quality and location as well as time. Successful microscopic analysis of the excised tissue requires high-quality frozen sections, which may be compromised if the specimen is too thin or too thick, frozen inadequately, or contains adipose tissue. Multifocal tumors – those located in more than one region – present an additional challenge, as the Mohs surgeon may successfully detect a clear margin around one tumor portion when others still exist elsewhere.[14] Furthermore, Mohs surgery, particularly when it involves several rounds of excisions, may be time-consuming.
In the periocular area, there exist several additional limitations in the use of Mohs surgery, particularly with regards to lesion extension and tumor type.[15] Extension of lesions to orbital fat may impede margin control as adipose tissue is known to compromise frozen sections and thereby hinders effective margin control.[14] Sebaceous cell carcinomas, due to their multicentricity and pagetoid spread, may also hinder accurate detection of negative margins.[16][17] Finally, in the context of periocular melanoma, as mentioned above, frozen section processing may compromise the microscopic morphology of the epidermis.[18] This may be resolved using Slow-Mohs, which involves multi-day processing of permanent sections instead of frozen sections (with the downside being multiple consecutive days of procedures for the patient) vs an alternative approach utilizing MART-1 immunohistochemical staining to maximize detection of atypical melanocytes.[18][19][4]
References
- ↑ Jump up to: 1.0 1.1 Golda N, Hruza G. Mohs micrographic surgery. Dermatol Clin. Jan 2023;41(1):39-47. doi:10.1016/j.det.2022.07.006
- ↑ Mohs FE. Mohs micrographic surgery. A historical perspective. Dermatol Clin. Oct 1989;7(4):609-11.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Etzkorn JR, Alam M. What is Mohs surgery? JAMA Dermatol. Jun 01 2020;156(6):716. doi:10.1001/jamadermatol.2020.0039
- ↑ Jump up to: 4.0 4.1 McInnis-Smith KM, Asamoah EM, Demer AM, Sharma K, Yu CY, Bradley EA, Tooley AA, Wagner LH. Mohs micrographic surgery with immunohistochemistry for the treatment of periocular melanoma in situ. Ophthalmic Plast Reconstr Surg. 2024. doi:10.1097/IOP.0000000000002729
- ↑ Young JN, Nguyen TA, Freeman SC, et al. Permanent section margin concordance after Mohs micrographic surgery with immunohistochemistry for invasive melanoma and melanoma in situ: a retrospective dual-center analysis. J Am Acad Dermatol 2023;88:1060–1065.
- ↑ Elgash M, Young J, White K, et al. An update and review of clinical outcomes using immunohistochemical stains in Mohs micrographic surgery for melanoma. Dermatol Surg 2024;50:9–15.
- ↑ Nosrati A, Berliner JG, Goel S, et al. Outcomes of melanoma in situ treated with Mohs micrographic surgery compared with wide local excision. JAMA Dermatol 2017;153:436–441.
- ↑ Ad Hoc Task Force, Connolly SM, Baker DR, et al. AAD/ACMS/ASDSA/ASMS 2012 Appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2012;67(4):531-550.
- ↑ Technology and Innovation in Learning Team at the University of Dundee School of Medicine. University of Dundee Mohs micrographic surgery conventional excision. Flickr. Published November 23, 2016. Accessed February 1, 2023. https://www.flickr.com/photos/138501603@N02/31088117871. Licensed under CC BY-NC-ND 2.0.
- ↑ Bittner GC, Cerci FB, Kubo EM, Tolkachjov SN. Mohs micrographic surgery: a review of indications, technique, outcomes, and considerations. An Bras Dermatol. 2021 May-Jun;96(3):263-277. doi: 10.1016/j.abd.2020.10.004.
- ↑ Muller FM, Dawe RS, Moseley H, Fleming CJ. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: Tissue-sparing outcome. Dermatol Surg. Sep 2009;35(9):1349-54. doi:10.1111/j.1524-4725.2009.01240.x
- ↑ van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs' micrographic surgery for basal cell carcinoma of the face: A randomised clinical trial with 10 year follow-up. Eur J Cancer. Nov 2014;50(17):3011-20. doi:10.1016/j.ejca.2014.08.018
- ↑ Demer AM, Vance KK, Cheraghi N, Reich HC, Lee PK. Benefit of Mohs Micrographic Surgery Over Wide Local Excision for Melanoma of the Head and Neck: A Rational Approach to Treatment. Dermatol Surg. 2019 Mar;45(3):381-389. doi: 10.1097/DSS.0000000000001715.
- ↑ Jump up to: 14.0 14.1 Rapini RP. Pitfalls of Mohs micrographic surgery. J Am Acad Dermatol. Apr 1990;22(4):681-6. doi:10.1016/0190-9622(90)70095-y
- ↑ Patel SY, Itani K. Review of eyelid reconstruction techniques after Mohs surgery. Semin Plast Surg. May 2018;32(2):95-102. doi:10.1055/s-0038-1642058
- ↑ Slutsky JB, Jones EC. Periocular cutaneous malignancies: A review of the literature. Dermatol Surg. Apr 2012;38(4):552-69. doi:10.1111/j.1524-4725.2012.02367.x
- ↑ Shields JA, Demirci H, Marr BP, Eagle RC, Shields CL. Sebaceous carcinoma of the ocular region: A review. Surv Ophthalmol. 2005;50(2):103-22. doi:10.1016/j.survophthal.2004.12.008
- ↑ Jump up to: 18.0 18.1 Harvey DT, Taylor RS, Itani KM, Loewinger RJ. Mohs micrographic surgery of the eyelid: An overview of anatomy, pathophysiology, and reconstruction options. Dermatol Surg. May 2013;39(5):673-97. doi:10.1111/dsu.12084
- ↑ El Tal AK, Abrou AE, Stiff MA, Mehregan DA. Immunostaining in Mohs micrographic surgery: A review. Dermatol Surg. Mar 2010;36(3):275-90. doi:10.1111/j.1524-4725.2009.01432.x