Minimally Invasive Glaucoma Surgery in Childhood Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Childhood glaucoma is a group of heterogeneous disorders characterized by elevated intraocular pressure (IOP) which if left untreated leads to glaucomatous optic neuropathy and visual impairment.[1] Angle-based surgery whether goniotomy or trabeculotomy is the main surgical management of primary congenital glaucoma.[2] Trabeculectomy and glaucoma drainage devices are usually reserved for refractory cases or secondary childhood glaucoma which is likely to fail angle surgery.[3][4]
Over the past decade, there has been growing interest among glaucoma specialists to perform minimally (or micro-) invasive glaucoma surgery (MIGS) mainly in mild to moderate glaucoma due to its high safety profile. The idea of MIGS as conjunctiva-sparing procedures is attractive to pediatric glaucoma specialists, however, there is limited evidence in the literature regarding its safety and efficacy in childhood glaucoma. In this article, we review the published literature on MIGS in the pediatric population
Kahook dual-blade
The Kahook dual-blade (KDB, New World Medical, Rancho Cucamonga, CA) was introduced in 2015 and designed to excise a TM strip rather than creating incising it as in conventional goniotomy. The KDB has a footplate that rests against the SC. As the device advances along the iridotrabecular angle, the sharp blades on either side of the device create two parallel incisions allowing for complete removal of the TM strip.
The use of KDB in the management of childhood glaucoma was first reported by Khouri and Wong[5] who reported successful IOP reduction in both eyes of an 11-month-old infant who underwent KDB ab-interno trabeculectomy for management of his glaucoma following cataract surgery. After a follow-up period of 7 to 10 weeks, the IOP was reduced from 35 mmHg and 52 mmHg to 17 mmHg and 18 mmHg in the right and left eyes, respectively.
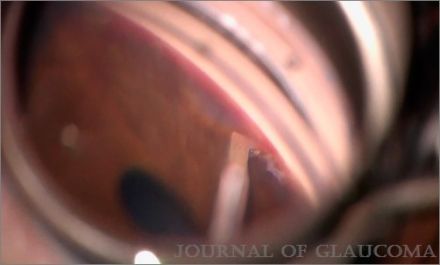
The largest to date study evaluating the efficacy of KDB in childhood glaucoma was conducted by Elhilail and colleagues.[6] They prospectively compared ab-interno trabeculectomy using KDB to traditional goniotomy using a 25-gauge irrigating needle in PCG patients. The study included 42 eyes (29 patients), of which 21 underwent KDB ab-interno trabeculectomy (Figure 1) and 21 underwent conventional goniotomy. There was a significant reduction in the mean IOP from baseline of 24.4±6.8 mmHg and 23.05±3.7 mmHg to 11.8±2.3 mmHg and 12.8±2.6 mmHg at the 12-months= follow-up visit in the KDB and the goniotomy groups, respectively. Success rate, defined as IOP ≤21 mmHg with or without glaucoma medications, was 57.1% in both groups at the 12-month follow-up visit. There was no statistically significant difference in the mean IOP reduction or the mean number of IOP lowering medications between both groups at any time point.[6]
Table 1 summarizes the studies evaluating the efficacy of KDB in childhood glaucoma.
| Study | Design | Number of eyes | Diagnosis | Preoperative IOP (mmHg) | Postoperative IOP (mmHg) | Follow-up |
|---|---|---|---|---|---|---|
| Elhilali et al.[6] (2021) | Prospective comparative study | 21 eyes | PCG | 24.4±6.8 (mean) | 11.8±2.3 (mean) | 12-months |
| Ibrahim et al.[7] (2021) | Case series | 3 eyes | PCG | Case 1: 36
Case 2 and 3: 24-28 |
Case 1: 14
Case 2 and 3: 24 |
Case 1: 4 months
Case 2 and 3: 6 months |
| Harvey and Schmitz[8] (2020) | Case report | 2 eyes | PCG | 43 OD
44 OS |
21 OD
34 OS |
3 months |
| Khouri et al.[9] (2019) | Case report | 1 eye | JOAG | 28 | 15 | 18 months |
| Khouri and Wong [5](2017) | Case report | 2 eyes | GFCS | 35 OD
52 OS |
17 OD
18 OS |
7-10 weeks |
| Reis et al.[10] (2022) | Case report | 1 eye | Angle-recession
glaucoma |
30 | 11 | 2 years |
Gonioscopy-assisted transluminal trabeculotomy
Gonioscopy-assisted transluminal trabeculotomy (GATT) was first described by Grover and colleagues in 2014.[11] The original technique was described as creating a nasal goniotomy incision through which an illuminated microcatheter or suture was introduced and advanced circumferentially through the lumen of SC. The microcatheter or the suture was then pulled out creating a circumferential trabeculotomy.[12]
The safety and efficacy of GATT were first demonstrated by Grover and colleagues[13] in a retrospective study including 14 eyes (4 PCG eyes and 10 JOAG eyes). All patients had a follow-up >12 months. They reported a statistically significant reduction in the mean IOP from 27.3 mmHg on 2.6 glaucoma medications preoperatively to 14.8 mmHg on 0.86 glaucoma medications postoperatively. The most common complication was hyphema which occurred in 5 eyes and cleared by 1 month postoperatively on conservative treatment.
A recent study conducted by Quan and colleagues[14] evaluated the risk factors of complications and failure after GATT in childhood glaucoma. The study included 74 eyes (57 patients) with a mean age of 7.1 years at the time of GATT. The failure rate, defined as IOP <5 mmHg or >21 mmHg on 2 consecutive visits, <20% IOP reduction, development of vision-threatening complications or need for further glaucoma surgery, was 48.6% at a median follow-up of 28.5 months. They reported a higher risk of failure in patients who had postoperative topical steroids (compared to non-steroidal anti-inflammatory drops), IOP spikes without hyphema, younger age, and partial trabeculotomy.
A prospective study by Shi and colleagues[15] included 70 JOAG eyes with a median age of 19.3 years at the time of surgery. There was a statistically significant reduction in the mean IOP from a baseline of 31.3±9.5 mmHg to 15.8±2.7 mmHg at 1-year postoperatively. They reported success rates, defined as a postoperative IOP of ≤ 21 mmHg and ≥20% IOP reduction without (complete success) or with (qualified success) glaucoma medications, of 91.4% (qualified success) and 74.3% (complete success).
A retrospective study by Shi and colleagues[16] compared the outcomes of GATT using a microcatheter to ab-externo microcatheter-assisted circumferential trabeculotomy (MAT) in 115 PCG eyes. They defined success as a postoperative IOP ≤ 21 mmHg and ≥30% IOP reduction with (qualified) or without (complete) glaucoma medications. There was no statistically significant difference in the rates of qualified (87.9% GATT vs 82.2% MAT) or complete success (81% GATT vs 73% MAT) between both groups. Elhusseiny and colleagues evaluated the effectiveness of GATT in 7 PCG eyes who had a history of failed glaucoma surgery. They reported a significant reduction of the mean IOP from 25.7±5.9 mmHg at baseline to a mean of 15.8±2.7 mmHg at 1-year postoperatively. At the one-year follow-up, none of the 7 eyes required further glaucoma intervention.[17]
Trab360
The Trab360 (Sight Sciences, Menlo Park, CA, USA) is a novel device that was introduced in 2015. It is designed to perform ab-interno circumferential trabeculotomy through a 1 mm corneal incision. The device has a bent needle tip and an internal nylon filament with a bulbous tip. After the SC is cannulated, the filament can be advanced by rotating the wheel on the handpiece and cutting through 180◦ of the TM. If the surgeon wants to treat the other 180◦, the device can be rotated, and the same steps are repeated in the other 180◦.
In childhood glaucoma, Areaux and colleagues[18] evaluated the efficacy of Trab360 in 46 eyes with a median age of 12 months at the time of surgery. The mean extent of the angle treated was 290◦. They reported a significant reduction in the median IOP from a baseline of 30 mmHg on a median of 2.5 glaucoma medications to a median of 18 mmHg on a median of 1 glaucoma medications. Out of 46 eyes, 40 underwent Trab360 as the initial glaucoma procedure and had a success rate, defined as IOP ≤ 24 mmHg with or without glaucoma medications and no further IOP lowering procedure or development of sight-threatening complications, of 70%. However, the success rate was higher in PCG compared to secondary glaucoma.[18][18]
OMNI surgical system
OMNI (Sight Sciences, Menlo Park, CA, USA) device is similar to Trab360TM with a sharp tip that incises the TM and then the microcatheter is advanced into the lumen of SC. However, on retraction, a considerable amount of viscoelastic material can be injected into the SC and distal collector channels (viscocanalostomy) which is thought to be helpful with post-TM resistance.
Porsia and Nicoletti[19] reported the use of the OMNI surgical system in the management of early-onset Sturge-Weber syndrome associated glaucoma in a 4-month-old girl’s right eye. The preoperative IOP was >30 mmHg and was reduced to 15 mmHg at 2-month and 18 mmHg at the 10-month follow-up visit.[19]
XEN gel stent
XEN gel stent (Allergan, Dublin, Ireland) is a glaucoma implant designed to drain the aqueous humor from the anterior chamber to the subconjunctival space. The tube is 6mm in length. The outer diameter of the stent is 150 um while the inner lumen is 45 um.
Limited evidence is currently available for the efficacy of XEN gel stent in the management of childhood glaucoma. The first report was published by Sousa and colleagues who described bilateral XEN gel stent implantation in a 14-year-old girl with steroid-induced ocular hypertension. The preoperative IOP was 40 mmHg right eye and 34 mmHg left eye on 4 anti-glaucoma medications. At the 6-month postoperative follow-up visit, the IOP was 14-16 mmHg right eye and 13-14 mmHg left eye without glaucoma medications.[20] Smith and colleagues[21] reported implantation of XEN gel stent in 3 pediatric glaucoma eyes. They reported IOP control over a follow-up period of 6-24 months.
Schellhase and colleagues[22] described a XEN gel stent implantation in a 1-year-old with pediatric glaucoma associated with Neurofibromatosis type 1. They reported that despite multiple revisions the IOP was not controlled, and the patient eventually underwent cyclophotocoagulation and loss of light perception
PreserFlo ab-externo microshunt
PreserFlo is a novel microshunt that is pending approval by the US FDA; however, it is CE-marked in Europe. The device shunts the aqueous from the anterior chamber to the subconjunctival space. The length of the device is 8.5 mm with a 350-um external lumen and 70-um internal lumen.
Several studies have evaluated its safety and efficacy in adult glaucoma. In childhood glaucoma, a recent prospective study by Brandt[23] reported promising results in 12 eyes (Figure 2). The success rate, defined as IOP reduction ≥ 25% with or without IOP-lowering medications and evidence of clinical response, in 9 eyes (75%) with a postoperative IOP of 11.0±2.5 mmHg in the success group. Another study by Burgos-Blasco and colleagues prospectively evaluated the outcomes of mitomycin-C augmented PreserFlo implantation in 14 childhood glaucoma patients after failed previous glaucoma surgeries. They reported that 86% of patients had at least 20% IOP reduction at 1-year postoperatively compared to the baseline. They did not report any severe adverse events or intraoperative complications in their cohort.[24]
Endoscopic cyclophotocoagulation
Several studies have evaluated the efficacy of endoscopic cyclophotocoagulation (ECP) in childhood glaucoma. The first study was conducted by Neely and Plager[25] including 36 eyes with primary or secondary childhood glaucoma. They reported a significant reduction in the mean IOP from 35.06±8.55 mmHg preoperatively to 23.63±11.07 at the last follow-up with a success rate of 34% after a single ECP procedure.
Another study by Carter and colleagues[26] included 34 eyes with glaucoma following cataract surgery who underwent ECP with a mean follow-up of 44.4 months. They reported a significant reduction in the mean IOP from 32.6 mmHg to 22.9 mmHg at the last follow-up.
The largest study to date was conducted by Glaser and colleagues[27] including 80 eyes with a median follow-up of 1.1 years. Multiple ECP sessions resulted in a success rate of 81% at 1-year, 49% at 3-years, and 34% at 5-years.
Conclusions
There is limited evidence regarding the efficacy of different MIGS procedures in childhood glaucoma. Most studies are limited by small sample sizes, retrospective nature, and short-term follow-up. GATT, Trab360, and PreserFlo results are encouraging, however, further studies are needed to better understand the safety and the long-term outcomes of these procedures
References
- ↑ Tam EK, Elhusseiny AM, Shah AS, Mantagos IS, VanderVeen DK. Etiology and outcomes of childhood glaucoma at a tertiary referral center [published online ahead of print, 2022 Apr 8]. J AAPOS. 2022;S1091-8531(22)00064-7.
- ↑ Mocan MC, Mehta AA, Aref AA. Update in Genetics and Surgical Management of Primary Congenital Glaucoma. Turk J Ophthalmol. 2019;49(6):347-355
- ↑ Neustein RF, Bruce BB, Beck AD. Primary Congenital Glaucoma Versus Glaucoma Following Congenital Cataract Surgery: Comparative Clinical Features and Long-term Outcomes. Am J Ophthalmol. 2016;170:214-222.
- ↑ Elhusseiny AM, VanderVeen DK. Outcomes of Glaucoma Drainage Devices in Childhood Glaucoma. Semin Ophthalmol. 2020;35(3):194-204.
- ↑ Jump up to: 5.0 5.1 Khouri AS, Wong SH. Ab Interno Trabeculectomy With a Dual Blade: Surgical Technique for Childhood Glaucoma. J Glaucoma. 2017;26(8):749-751.
- ↑ Jump up to: 6.0 6.1 6.2 Elhilali, Hala M. MD; El Sayed, Yasmine M. MD; Elhusseiny, Abdelrahman M. MD; Gawdat, Ghada I. MD Kahook Dual Blade Ab-interno Trabeculectomy Compared With Conventional Goniotomy in the Treatment of Primary Congenital Glaucoma: 1-Year Results, Journal of Glaucoma: June 2021 - Volume 30 - Issue 6 - p 526-531
- ↑ Ibrahim LF, Silva SAR, Prata TS, Kanadani FN. Short-term results of ab-interno trabeculotomy using Kahook Dual Blade in patients with primary congenital glaucoma. Arq Bras Oftalmol. 2021;84(4):380-382.
- ↑ Harvey MM, Schmitz JW. Use of ab interno Kahook Dual Blade trabeculectomy for treatment of primary congenital glaucoma. Eur J Ophthalmol. 2020;30(1):NP16-NP20.
- ↑ Khouri AS, Zhu Y, Sadek H. Ab interno trabeculectomy with the dual blade in juvenile open-angle glaucoma. Eur J Ophthalmol. 2021;31(2):NP43-NP45.
- ↑ Reis LS, Arancibia AEL, Prata TS, Kanadani FN. Ab-interno trabeculotomy with Kahook dual blade in secondary traumatic glaucoma in a child. Am J Ophthalmol Case Rep. 2022;25:101354. Published 2022 Jan 26.
- ↑ Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855-861.
- ↑ Aboalazayem F, Elhusseiny AM, El Sayed YM. Gonioscopy-Assisted Transluminal Trabeculotomy; A Review [published online ahead of print, 2022 May 28]. Curr Eye Res. 2022;1-26.
- ↑ Grover DS, Smith O, Fellman RL, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol. 2015;99(8):1092-1096.
- ↑ Quan AV, Chen J, Wang YE, et al. Factors Associated with Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) Complications and Failure in Children [published online ahead of print, 2022 May 9]. Am J Ophthalmol. 2022;S0002-9394(22)00177-5.
- ↑ Shi Y, Wang H, Oatts JT, et al. A Prospective Study of Intraocular Pressure Spike and Failure After Gonioscopy-Assisted Transluminal Trabeculotomy in Juvenile Open-Angle Glaucoma: A Prospective Study of GATT in JOAG. Am J Ophthalmol. 2022;236:79-88.
- ↑ Shi Y, Wang H, Oatts J, et al. Ab interno vs ab externo microcatheter-assisted trabeculotomy for primary congenital glaucoma with clear cornea. Clin Exp Ophthalmol. 2020;48(9):1201-1209.
- ↑ Elhusseiny AM, Aboulhassan RM, El Sayed YM, Gawdat GI, Elhilali HM. Gonioscopy-Assisted Transluminal Trabeculotomy following Failed Glaucoma Surgery in Primary Congenital Glaucoma: One-Year Results. Case Rep Ophthalmol Med. 2023;2023:6761408.
- ↑ Jump up to: 18.0 18.1 18.2 Areaux RG Jr, Grajewski AL, Balasubramaniam S, et al. Trabeculotomy Ab Interno With the Trab360 Device for Childhood Glaucomas. Am J Ophthalmol. 2020;209:178-186.
- ↑ Jump up to: 19.0 19.1 Porsia L, Nicoletti M. Combined Viscodilation of Schlemm's Canal and Collector Channels and 360° Ab-Interno Trabeculotomy for Congenital Glaucoma Associated with Sturge-Weber Syndrome. Int Med Case Rep J. 2020;13:217-220.
- ↑ Sousa DC, Leal I, Abegão Pinto L. Steroid-induced protracted severe ocular hypertension in a 14-year-old girl. BMJ Case Rep. 2018;2018:bcr2018225244.
- ↑ Smith OU, Grover DS, Emanuel ME, Godfrey DG, Fellman RL. XEN Gel Stent in Pediatric Glaucoma. J Glaucoma. 2020;29(4):e19-e22.
- ↑ Schellhase H, Fuest M, Kuerten D, Walter P, Plange N. Failure of XEN Gel Stent Implantation as a Stand-Alone Procedure in Congenital Glaucoma: Case Report of Secondary Congenital Glaucoma in Neurofibromatosis Type 1. Case Rep Ophthalmol Med.
- ↑ Brandt JD. Use of a Novel Microshunt in Refractory Childhood Glaucoma: Initial Experience in a Compassionate Use/Early Access Cohort [published online ahead of print, 2022 Mar 26]. Am J Ophthalmol. 2022;239:223-229.
- ↑ Burgos-Blasco B, García-Feijóo J, Gines-Gallego C, et al. Efficacy and safety of the PreserFlo implant with mitomycin C in childhood glaucoma after previous failed glaucoma surgeries. Graefes Arch Clin Exp Ophthalmol. 2023;261(5):1349-1357.
- ↑ Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS. 2001;5(4):221-229.
- ↑ Carter BC, Plager DA, Neely DE, Sprunger DT, Sondhi N, Roberts GJ. Endoscopic diode laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. J AAPOS. 2007;11(1):34-40.
- ↑ Glaser TS, Mulvihill MS, Freedman SF. Endoscopic cyclophotocoagulation (ECP) for childhood glaucoma: a large single-center cohort experience. J AAPOS. 2019;23(2):84.e1-84.e7.