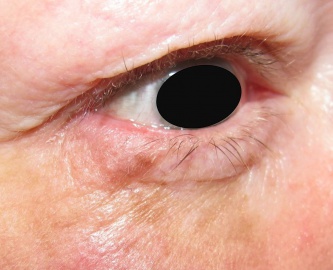
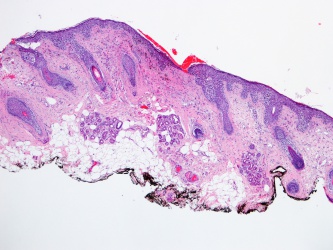
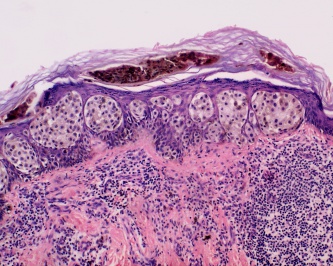
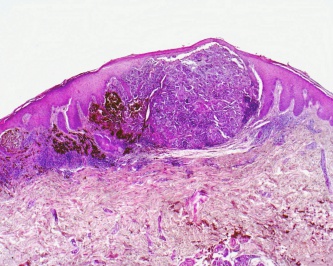
Malignant Melanoma of the Eyelid
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
ICD9 172.1 Malignant Melanoma of Skin of Eyelid including Canthus
ICD10 Codes:
- C43.10 Malignant Melanoma of Unspecified Eyelid, including Canthus
- C43.11 Malignant Melanoma of Right Eyelid, including Canthus
- C43.12 Malignant Melanoma of Left Eyelid, including Canthus
- D03.10 Melanoma in situ of Unspecified Eyelid, including Canthus
- D03.11 Melanoma in situ of Right Eyelid, including Canthus
- D03.12 Melanoma in situ of Left Eyelid, including Canthus
Disease
Cutaneous malignant melanoma of the eyelid skin arises from the malignant proliferation of melanocytes. It can arise de novo or from a pre-existing nevus.[1]
Epidemiology
Primary melanomas of the eyelid skin are rare. They account for <1% of all cutaneous malignant melanomas, <7% of head and neck melanomas, and about 1% of malignant eyelid tumors. The peak incidence of any head and neck melanoma is in 50-80 year olds, approximately 20 years later than cutaneous melanomas of other sites. The most common location of eyelid melanomas is the lower eyelid, where it is approximately 2.6 times more likely to occur than the upper eyelid. [2]
Etiology and Risk Factors
As with cutaneous melanomas of other sites, eyelid melanomas are usually a result of DNA damage from exposure to UV light, particularly UVB (290-320nm). In addition to UV exposure, other common risk factors include fair skin, the presence of dysplastic or congenital nevi, family history of melanoma, and older age. [1][2]
Pathology
Cutaneous malignant melanoma results from abnormal proliferation of atypical melanocytes derived from the epidermis.
The four major subtypes of melanoma include the following: [1][2]
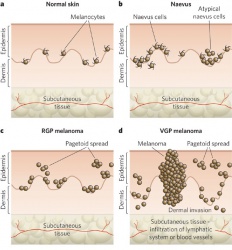
- Lentingo maligna melanoma - This is most common subtype seen on the eyelid skin, but is the least common overall. It is characterized by atypical, mostly spindle-shaped melanocytes at the basal epidermis and can also extend to adnexal structures. These often arise from sites of actinic damage and areas of solar elastosis. Lentigo maligna melanoma may also exhibit pagetoid growth that can ultimately involve all layers of the epidermis and invade vertically into the dermis.
- Superficial Spreading melanoma - This is characterized by scattered or nests of epithelioid cells throughout the epidermis, and can exhibit vertical growth with invasion of the dermis.
- Nodular melanoma - This is predominantly characterized by a vertical growth pattern, usually composed of epithelioid type cells.
- Acral Lentiginous melanoma - Occurs on acral surfaces such as the palms, soles, under nails, and oral mucosa. This darkly pigmented, irregular, flat lesion is characterized by growth of atypical melanocytes arranged mostly as single units at the dermal-epidermal junction. [3]
Pathophysiology
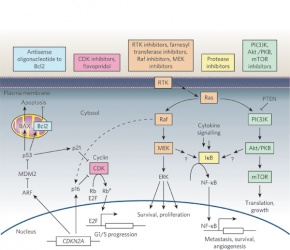
Transformation of a normal melanocyte occurs by accumulation of genetic and molecular alterations. The chromosomes most frequently showing aberrations include 1, 6, 7, 9-11, 17, and 20. Key proliferative pathways that are altered involve the MAPK signal transduction pathway (RAS-RAF-MEK-ERK). Activation of this pathway has been shown to induce cell proliferation and survival. In addition, dysregulation of apoptotic pathways that include tumor suppressor genes like CDKN2A, PT53, or PTEN also contribute to the development of melanomas. [1]
Malignant melanoma first begins in a radial growth phase that is characterized by superficial -intraepidermal or superficial dermal- growth. These tumors are typically not associated with metastatic spread and lack mitotic figures. They can evolve to display aggressive vertical growth that invades the dermis. This is where mitotic figures become more prominent and is associated with a greater chance of ulceration. Malignant melanoma can also exhibit direct extension to surrounding tissues, such as the conjunctiva, or they can metastasize hematogenously or via lymphatics. [2]
Diagnosis
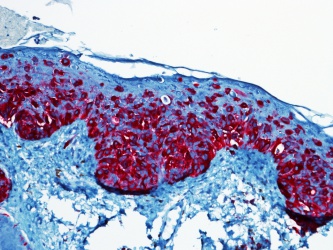
The diagnosis of cutaneous malignant melanoma of the eyelid is suspected clinically and confirmed histologically.
Suspicious lesions can be identified using the classic "ABCD" method of evaluation (Asymmetry, Border irregularity, Color variation, Diameter). Examination should also include palpation for regional lymphadenopathy.
Suspicious lesions can present along a spectrum ranging from flat tan macules with irregular borders, to nodular or elevated lesion with a combination of colors that may include tan, black, gray, pink, blue, or white. Melanoma can also present as amelanotic lesions, thus making it more difficult to identify, especially in fair skinned people.[2] In such cases, it is important to look for other clues such as erythema, scaling, or irregular borders, or evaluate by dermoscopy.[4]
Immunohistochemical Stains
S100 is a calcium-binding protein is present in the nucleus and cytoplasm of melanocytes. This stain is associated with a sensitivity of 97-100%, however the specificity is more limited (75-87%) as this protein is also identified in nerve sheath cells, myoepithelial cells, adipocytes, chondrocytes, and Langerhans cells. [5]
MelanA/MART-1 is Melanoma antigen recognized by T-cells (MART-1). It is expressed by most melanocytic lesions, and detected by Melan-A antibody. Sensitivity is 75-92%, and specificity is 95-100%.[5]
HMB-45 is a marker for the cytoplasmic premelanosomal glycoprotein gp100. The sensitivity ranges from 69-93% by various studies, but has nearly 97% specificity.[6] The protein has also been expressed in meningeal melanocytoma, clear cell sarcoma, some breast cancers, and some renal cell carcinomas, however these are histologically different from melanomas.[5]
Sentinel Lymph Node Biopsy
SLN Biopsy helps guide staging which is important for the patient's prognosis and therapeutic options.
According to the National Comprehensive Cancer Network recommendations (version 2.2023), SLN biopsy is not recommended for a primary melanoma less than 0.8mm thick, unless there is significant uncertainty about the adequacy of microstaging. In general, for melanomas less than 1.0mm thick, there is little consensus about high risk features, and SLN biopsy for melanomas 0.8mm-1.0mm should be considered on an individual basis. The presence of ulceration should prompt consideration for SNL biopsy for lesions of any depth, even those <0.8mm. Melanomas greater than 1.0mm thick without clinically evident nodes should be offered SLN biopsy, but the procedure may be deferred based on patient comorbidities or personal preference.
Differential diagnosis
- Seborrheic keratosis
- Actinic keratosis
- Nevus
- Verruca
- Basal cell carcinoma
- Squamous cell carcinoma
- Sebaceous gland carcinoma
- Merkel cell tumor
- Metastasis
Staging
NCCN Guidelines (version 2.2023) for cutaneous melanomas staging.[7]
T refers to thickness and level of invasion, and is further classified by presence or absence of ulceration.
- Stage 0 - melanoma in situ
- Stage IA (T1a) (<0.8 mm thick, no ulceration)
- Stage IB (T1b) (<0.8 mm thick with ulceration or 0.8–1.0 mm thick ± ulceration)
- T1a < 0.8 mm, without ulceration
- T1b < 0.8 mm with ulceration 0.8–1.0 mm, with or without ulceration
- T2a > 1.0–2.0 mm, without ulceration
- T2b > 1.0–2.0 mm, with ulceration
- T3a > 2.0–4.0 mm, without ulceration
- T3b > 2.0–4.0 mm, with ulceration
- T4a > 4.0 mm, without ulceration
- T4b > 4.0 mm, with ulceration
N refers to involvement of lymph nodes
- N0 = 0 metastatic nodes
- N1 = 1 metastatic node
- N2 = 2-3 metastatic nodes
- N3 = 4+ metastatic nodes, or in transit metastases/satellites with metastatic nodes
N is further differentiated by nodal metastatic burden for N1-N3
- a: micrometastasis
- b: macrometastasis
For N3, there is the additional category:
- c: In transit metastases/satellites without metastatic nodes
M refers to metastasis
- M0 = No distant metastases
- M1a = Distant skin, subcutaneous, or nodal metastases with normal serum LDH
- M1b = Lung metastases with normal serum LDH
- M1c = All other visceral metastases with normal serum LDH or with any distant metastasis and elevated serum LDH
Management
Surgical excision with wide margins is the mainstay of early stage melanoma management. For melanoma in-situ (Stage 0), Mohs micrographic surgery is the treatment of choice over surgical excision, with recurrence rates reported as low as 0-3.6% and 6-20%, respectively.[8] Paraffin-embedded sections remain the gold standard over frozen sections for melanocytic lesions, as the latter can lead to more artifact making it more difficult to identify background actinic changes from melanoma. The major disadvantage of Mohs is that many techniques described in literature are staged and often carried out over weeks.[9]The MART1 immunostaining technique allows for improved melanoma detection on frozen sections to allow for a more standard single-day Mohs excision. [10]
According to National Comprehensive Cancer Network, the treatment for Stage 0, IA, and IB (and < 0.8 mm thick) is wide local excision (10 mm margins). Generally, routine lab work or imaging is not indicated except when associated with specific complaints. Stage IA, IB, and II can consider SLN biopsy on an individual basis, treat with wide local excision and can observe or consider a clinical trial. Routine labs or imaging is again not recommended unless to evaluate a specific sign or symptom. Melanomas > 2.0 mm thick should have 20mm margins. Patients in stage III with a positive SLN biopsy or clinically positive nodes should be considered for baseline imaging for further staging and to evaluate specific signs/symptoms. These patients should undergo excision and complete lymph node dissection, followed by observation, a clinical trial, or interferon alpha as adjuvant therapy. Radiation therapy to the nodal basin in some patients can also be considered. Patients with stage IV disease should undergo biopsy for genetic testing, labs to check LDH, and imaging. Immune check point inhibitors, including those targeting programmed cell death-1 (PD-1) including pembrolizumab (Ketruda) and nivolumab (Opdivo), have demonstrated good efficacy in treating some cases of locally advanced and metastatic eyelid and conjunctival melanomas, and may be used as primary treatment, neoadjuvantly or for palliation.[11] [12]
Prognosis
Significant risk factors include tumor thickness, ulceration, and mitotic rate. The 10 year survival rate ranges as high as 93% for stage IA to 39% for stage IIC melanomas. Stage IIIA melanoma has a 10 year survival rate of approximately 68%, while it is 24% for stage IIIC. Stage IV 10 year survival rate is 10-15%, depending on location of metastasis and LDH levels.[13][14]
Additional Resources
- Porter D, DeAngelis KD. Mohs Surgery. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/treatments/mohs-surgery-list. Accessed March 19, 2019.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1. Millman et al. The molecular genetics of eyelid tumors: recent advances and future directions. Arch Clin Exp Ophthalmol 2013; 251:419-433.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Boulos PR, Rubin PA. Cutaneous melanomas of the eyelid. Semin Ophthalmol. 2006 Jul-Sep;21(3):195-206.
- ↑ Bravo Puccio F1, Chian C. Acral junctional nevus versus acral lentiginous melanoma in situ: a differential diagnosis that should be based on clinicopathologic correlation. Arch Pathol Lab Med. 2011 Jul;135(7):847-52.
- ↑ Sbano P, Nami N, Grimaldi L, Rubegni P. True amelanotic melanoma: the great masquerader. J Plast Reconstr Aesthet Surg. 2010 Mar;63(3):e307-8
- ↑ Jump up to: 5.0 5.1 5.2 Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008 May;35(5):433-44.
- ↑ Sheffield MV1, Yee H, Dorvault CC, Weilbaecher KN, Eltoum IA, Siegal GP, Fisher DE, Chhieng DC. Comparison of five antibodies as markers in the diagnosis of melanoma in cytologic preparations. Am J Clin Pathol. 2002 Dec;118(6):930-6.
- ↑ NCCN Guidelines Version 2.2023 Melanoma: Cutaneoushttps://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- ↑ National Comprehensive Cancer Network. Available online:http://www.nccn.org
- ↑ Dawn et al. Mohs Surgery for the Treatment of Melanoma in Situ: A Review. Dermatol Surg. 2007 Apr;33(4):395-402.
- ↑ Valentín-Nogueras SM, Brodland DG, Zitelli JA, González-Sepúlveda L, Nazario CM. Mohs Micrographic Surgery Using MART-1 Immunostain in the Treatment of Invasive Melanoma and Melanoma In Situ. Dermatol Surg. 2016 Jun;42(6):733-44. doi: 10.1097/DSS.0000000000000725. PMID: 27158886; PMCID: PMC5422990.
- ↑ Lu JE, Chang JR, Berry JL, In GK, Zhang-Nunes S. Clinical Update on Checkpoint Inhibitor Therapy for Conjunctival and Eyelid Melanoma. Int Ophthalmol Clin. 2020 Spring;60(2):77-89. doi: 10.1097/IIO.0000000000000308. PMID: 32205655; PMCID: PMC8059069.
- ↑ Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Medicine 2016; 5( 7): 1481– 1491
- ↑ Charles M. Balch, et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J Clin Oncol. Dec 20, 2009; 27(36): [[1]].
- ↑ American Cancer Society. Available online: http://www.cancer.org/cancer/skincancer-melanoma/detailedguide/melanoma-skin-cancer-survival-rates