Lotilaner
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Overview
Lotilaner ophthalmic solution 0.25% (Xdemvy; Tarsus Pharmaceuticals, Inc.) was approved in 2023 in the United States as a treatment for blepharitis secondary to Demodex mite infestation.
Background
Demodex blepharitis (DB) is the most common form of blepharitis, affecting nearly 25 million people in the United States.[1] DB accounts for about 60% to 70% of all cases of blepharitis.[2][3][4] DB is characterized by chronic inflammation of the eyelid margins secondary to Demodex mite infestation of eyelash follicles and the meibomian glands.[5][6] Patients affected by this condition suffer from a range of symptoms that may reduce their quality of life, including pruritis of the eyelid, dryness, ocular irritation, and blurry vision. As DB progresses to a more advanced stage, patients may begin to experience superficial corneal neovascularization, opacity, and scarring, which can cause permanent visual impairment.[7]
Prior to 2023, treatment options for DB were limited to symptomatic therapies such as warm compresses, lid scrubs, tea tree oil (with the active ingredient, 4-terpineol), hypochlorous acid, off-label antibiotics, or steroids. For more severe cases, mechanical debridement of lid margins may be performed.[8] Despite providing symptomatic relief, none of these treatments would result in the direct eradication of Demodex mites responsible for the disease process. In July of 2023, the FDA approved lotilaner solution 0.25% (Xdemvy) ophthalmic solution as the first pharmaceutical therapy for the treatment of DB by elimination of the mites.
Disease Entity
DB arises due to an infestation of Demodex mites, which are the most prevalent ectoparasites found in humans.[6][9] Demodex mites can be divided into several species, and of these two infect humans. Demodex folliculorum inhabits the eyelash and the infundibulum of the follicles. Demodex brevis inhabits sebaceous and meibomian glands.[8] [10] Demodex mites lack internal digestion mechanisms and instead secrete proteases and lipases that externally digest healthy epithelial cells and sebum within the eyelid.[11] While it was once believed Demodex mites lack excretory organs, causing an excess of undigested material which is eventually pushed out of the follicle and deposited at the eyelash base[8], this has been disproven with the discovery of an anus on Demodex mites[12].
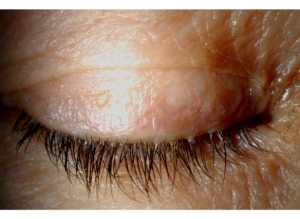
The accumulation of cylindrical sleeve-like debris, known as collarettes, at the base of the lashes are pathognomonic for a diagnosis of DB (Figure 1). Demodex collarettes are composed of partially digested epithelial cells, mite waste, and eggs.[13] Collarettes can be readily distinguished by an eye care professional by directing the slit lamp toward the upper lid margin and advising patients to gaze downward, ensuring a clear visibility of the foundation of the upper eyelashes.[14] Other clinical signs of Demodex blepharitis include erythema and edema along the lid margins, keratitis, misalignment or shedding of eyelashes, impairment of the meibomian glands, and disturbances in the tear film.[15]
Mechanism of Action
Lotilaner is an isoxazoline that selectively inhibits the gamma-aminobutyric acid (GABA)-gated chloride channels of insects and acari.[16] GABA-mediated chloride influx results in hyperpolarization of the cellular membrane, generating an inhibitory postsynaptic potential and consequently decreasing the probability of reaching the threshold for an action potential. Demodex mites and other acari possess GABA-gated chloride channels that are expressed in the central nervous system and at peripheral neuromuscular junctions, where they generate inhibitory potentials to promote muscular relaxation.[16] By inhibiting GABA-gated chloride channels, it is suspected that lotilaner is therapeutically beneficial to the treatment of DB by inducing a spastic paralysis, causing the starvation and death of exposed mites.[16]
Clinical Trials
One phase 2b/3 clinical trial (Saturn-1) and a second phase 3 clinical trial (Saturn-2) were conducted with a combined total of 833 patients to evaluate the efficacy and safety profile of lotilaner in treating DB.[14][17] Both clinical trials were 6-week, randomized, multicenter, double-masked, vehicle-controlled studies where patients with DB were randomized into two groups in a 1:1 ratio where they received either lotilaner ophthalmic solution 0.25% or a vehicle solution twice daily in each eye.[14][17]
The efficacy of lotilaner ophthalmic solution in treating DB in the Saturn-1 and Saturn-2 trials was measured based on the proportion of patients with either clinically meaningful collarette cure (≤ 10 lashes with collarettes-labeled as Grade 1) or complete collarette cure (<2 lashes with collarettes-labeled as Grade 0) on day 43 (Table 1).[14][17]
| Lotilaner Group | Vehicle Control Group | |||||
| Saturn-1 | Saturn-2 | Total | Saturn-1 | Saturn-2 | Total | |
| Clinically Meaningful Collarette Cure | 81.3% | 89.1% | 83.6% | 23.0% | 33.0% | 27.9% |
| Complete Collarette Cure | 44.0% | 56.0% | 49.8% | 7.4% | 12.5% | 9.9% |
The combined results of the Saturn-1 and Saturn-2 trials demonstrate that by day 43, 83.6% of patients being treated with lotilaner and 27.9% of patients in the vehicle control group reached clinically meaningful collarette cure.[14][17] In addition to the reduction of collarettes, the secondary endpoints measured included erythema cure and mite eradication. Lotilaner solution is effective at reducing erythema secondary to DB with 31.1% of patients in the lotilaner group of the Saturn-2 trial reaching erythema cure (erythema score of 0 for the upper eyelid of the analysis eye) by day 43.[14] Further investigations are needed to assess long term efficacy.
Contraindications and Adverse Events
The use of lotilaner for DB has no current contraindications. Lotilaner has not been studied in pregnant, breastfeeding, or pediatric populations. The most common adverse effect observed with lotilaner ophthalmic solution is pain or burning sensation at the site of installation which was reported by 8%-12% of patients.[14][17] Other less common adverse effects that were observed include dry eye, reduced visual acuity, chalazion, conjunctival hyperemia, eyelid pruritis, photophobia, visual impairment, instillation site irritation, and vital dye staining of the cornea (Table 2).[14] No clinically significant effects of lotilaner were observed by day 43 on corrected distance visual acuity, corneal staining, intraocular pressure, slit lamp biomicroscopy, endothelial cell density, dilated fundus examination, or systemic laboratory panel values.[14] Further investigations are needed to assess long term adverse effects.
| Percentage of Patients Experiencing Adverse Effect | ||
| Adverse Effect | Lotilaner Group | Vehicle Control Group |
| Installation Site Pain | 7.9 | 6.7 |
| Dry Eye | 1.5 | 0.5 |
| Reduced Visual Acuity | 0.5 | 1.4 |
| Chalazion | 1.0 | 0 |
| Conjunctival Hyperemia | 1.0 | 0 |
| Eyelid Pruritis | 0.5 | 0.5 |
| Photophobia | 1.0 | 0 |
| Visual Impairment | 0 | 1.0 |
| Instillation Site Irritation | 1.0 | 0 |
| Vital Dye Staining Cornea | 1.0 | 0 |
Dosing, Administration, and Preparation
Lotilaner is formulated as an ophthalmic solution containing 0.25% lotilaner (2.5 mg/mL).[18] Current prescribing guidelines recommend one drop of lotilaner to be administered twice a day to each eye for 6 weeks.[18] If a patient must utilize another ophthalmic solution while being treated, other medications should be administered at least 5 minutes apart from lotilaner.[18] If a dose is missed, patients may resume with the next scheduled dose.[18] Lotilaner ophthalmic solution contains potassium sorbate, a compound which may lead to the discoloration of contact lenses.[19] Consequently, lenses should be removed prior to administration of lotilaner ophthalmic solution, and be reinserted no sooner than 15 minutes after administration.
0.25% lotilaner ophthalmic solution has a wholesale acquisition cost of $1850 per prescription as of August 2023.[20] Additional information on preparation and administration can be found on the FDA label.
Patient Selection
The potential for adverse effects of lotilaner treatment in breast-feeding women has not been fully elucidated. Systemic exposure to lotilaner six weeks after administration is low and is >99% plasma protein bound; however, there is no data on the presence of 0.25% lotilaner ophthalmic solution in human milk.[18] It is unknown whether there are effects on the production of milk or on infants consuming breast milk. When deciding to prescribe lotilaner to breast-feeding women, it is important to consider the clinical necessity of treatment with lotilaner, the health benefits of breast-feeding, and the potential for adverse effects on children receiving milk.[18]
There are no differences in the safety or efficacy of treating DB with lotilaner observed between elderly and younger adults; however, safety and efficacy profiles have not yet been established for patients below the age of 18 years.[18] While there is a need for additional data on the effects of lotilaner on potentially vulnerable populations, ongoing clinical trials have begun to compare different dosages of lotilaner in ophthalmic solution and continue to further illuminate the safety and efficacy of this novel treatment.
Conclusions
Lotilaner ophthalmic solution 0.25% is the first FDA approved drug used to treat chronic blepharitis secondary to Demodex mite infestation. Two clinical trials have supported lotilaner to be safe and effective at curing collarettes and erythema in patients with DB. Additional studies are required to assess long term efficacy and adverse effects.
References
- ↑ Rhee MK, Yeu E, Barnett M, Rapuano CJ, Dhaliwal DK, Nichols KK, et al. Demodex Blepharitis: A Comprehensive Review of the Disease, Current Management, and Emerging Therapies. Eye Contact Lens 2023;49:311-318.
- ↑ Trattler W, Karpecki P, Rapoport Y, Sadri E, Schachter S, Whitley WO, et al. The Prevalence of Demodex Blepharitis in US Eye Care Clinic Patients as Determined by Collarettes: A Pathognomonic Sign. Clin Ophthalmol 2022;16:1153-1164.
- ↑ Biernat MM, Rusiecka-Ziółkowska J, Piątkowska E, Helemejko I, Biernat P, Gościniak G. Occurrence of Demodex species in patients with blepharitis and in healthy individuals: a 10-year observational study. Jpn J Ophthalmol 2018;62:628-633.
- ↑ Teo A, Rosenberg E, Jacobson A. Prevalence of Demodex Colonization in Patients Presenting to an Outpatient Clinic. Investigative Ophthalmology & Visual Science 2021;62:1236-1236.
- ↑ Cheng AM, Hwang J, Dermer H, Galor A. Prevalence of Ocular Demodicosis in an Older Population and Its Association With Symptoms and Signs of Dry Eye. Cornea 2021;40:995-1001.
- ↑ Jump up to: 6.0 6.1 Zhang AC, Muntz A, Wang MTM, Craig JP, Downie LE. Ocular Demodex: a systematic review of the clinical literature. Ophthalmic Physiol Opt 2020;40:389-432.
- ↑ Zhang XB, Ding YH, He W. The association between demodex infestation and ocular surface manifestations in meibomian gland dysfunction. Int J Ophthalmol 2018;11:589-592.
- ↑ Jump up to: 8.0 8.1 8.2 Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom (Auckl) 2018;10:57-63.
- ↑ Eberhardt M, Rammohan G. Blepharitis. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC., 2023.
- ↑ Cheng AM, Sheha H, Tseng SC. Recent advances on ocular Demodex infestation. Curr Opin Ophthalmol 2015;26:295-300.
- ↑ Nicholls SG, Oakley CL, Tan A, Vote BJ. Demodex species in human ocular disease: new clinicopathological aspects. Int Ophthalmol 2017;37:303-312.
- ↑ Gilbert Smith, Alejandro Manzano-Marín, Mariana Reyes-Prieto, Cátia Sofia Ribeiro Antunes, Victoria Ashworth, Obed Nanjul Goselle, Abdulhalem Abdulsamad A Jan, Andrés Moya, Amparo Latorre, M Alejandra Perotti, Henk R Braig, Human Follicular Mites: Ectoparasites Becoming Symbionts, Molecular Biology and Evolution, Volume 39, Issue 6, June 2022, msac125, https://doi.org/10.1093/molbev/msac125
- ↑ Gonzalez-Salinas R, Yeu E, Holdbrook M, Baba SN, Ceballos JC, Massaro-Corredor M, et al. Safety and Efficacy of Topical Lotilaner Ophthalmic Solution 0.25% for the Treatment of Demodex Blepharitis: A Pilot Study. J Ophthalmol 2021;2021:3862684.
- ↑ Jump up to: 14.0 14.1 14.2 14.3 14.4 14.5 14.6 14.7 14.8 Gaddie IB, Donnenfeld ED, Karpecki P, Vollmer P, Berdy GJ, Peterson JD, et al. Lotilaner Ophthalmic Solution 0.25% for <em>Demodex</em> Blepharitis: Randomized, Vehicle-Controlled, Multicenter, Phase 3 Trial (Saturn-2). Ophthalmology 2023;130:1015-1023.
- ↑ Rabensteiner DF, Aminfar H, Boldin I, Nitsche-Resch M, Berisha B, Schwantzer G, et al. Demodex Mite Infestation and its Associations with Tear Film and Ocular Surface Parameters in Patients with Ocular Discomfort. Am J Ophthalmol 2019;204:7-12.
- ↑ Jump up to: 16.0 16.1 16.2 Toutain CE, Seewald W, Jung M. The intravenous and oral pharmacokinetics of lotilaner in dogs. Parasit Vectors 2017;10:522.
- ↑ Jump up to: 17.0 17.1 17.2 17.3 17.4 Yeu E, Wirta DL, Karpecki P, Baba SN, Holdbrook M. Lotilaner Ophthalmic Solution, 0.25%, for the Treatment of Demodex Blepharitis: Results of a Prospective, Randomized, Vehicle-Controlled, Double-Masked, Pivotal Trial (Saturn-1). Cornea 2023;42:435-443.
- ↑ Jump up to: 18.0 18.1 18.2 18.3 18.4 18.5 18.6 NDA 217603 Highlights of Prescribing Information. In: Administration USFaD (ed), 2023.
- ↑ Gurnan B, Kaur K. Contact Lens–Related Complications. StatPearls 2023.
- ↑ Tarsus XDEMVY™ FDA Approval Conference Call. 2023.