Laser Trabeculotomy in Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Over the past several years, minimally invasive glaucoma surgeries (MIGS) have become integral to glaucoma management because of their high safety profile. With early encouraging results, laser trabeculotomies (or more accurately trabeculostomies)—whether performed with an excimer laser (ELT) or a femtosecond laser (FLIGHT)—have a promising role in the MIGS armamentarium. Selective laser trabeculoplasty increases aqueous drainage by inducing biological effects in the trabecular meshwork (TM) cells. In contrast, laser trabeculostomies increase aqueous drainage by creating channels through the TM into the Schlemm’s canal. [1] [2] It should be noted that ELT is an ab-interno procedure, and the eye has to be opened. In contrast, the FLIGHT procedure is a non-invasive procedure delivering the laser through the cornea without opening the eye.
Excimer laser trabeculostomy
Idea
Excimer laser trabeculostomy (ELT) creates holes/channels between the anterior chamber and Schlemm's canal using a 308 nm xenon chloride excimer laser. The ab-interno, non-thermal method minimizes scar formation and inflammatory reactions while protecting the integrity of surrounding tissues and collector channels. [3] ELT was performed as a standalone procedure and combined with phacoemulsification. [4] [5][6] Nevertheless, recent long-term studies have shown that repeat sessions are only occasionally required. [7]
Effectiveness and safety
Evidence from multiple studies (summarized in Table 1) supports the safety and effectiveness of the ELT procedure. The first study on human eyes was conducted in 1996 by Vogel and colleagues. [8] The preliminary results showed intraocular pressure (IOP) reduction of 11 mmHg over a 5-month follow-up period in four out of six eyes. In another study by the same investigators with a follow-up duration of 7 months, the median IOP was reduced by 7 mmHg in 22 out of 27 eyes. Although 12 eyes still required medication, a lower medication burden was needed, and the patients maintained reduced IOP levels.[9]
A subsequent study by Stodtmeister and colleagues [4], including 166 eyes and a follow-up duration of 1 year (130 eyes only), found that the mean IOP decreased from 26.4±6.2 mmHg at baseline to 16.9±4.7 mmHg (p<0.00001) at 1-year follow-up and no change in the number of medications than preoperative. In another study including 46 eyes and a follow-up duration of 5 years (28 eyes only), the mean IOP decreased from 25.5±6.3 at baseline to 15.9±3.0 mmHg at the 5-year follow-up visit (p<0.001) and the mean number of IOP-lowering medications decreased from 1.9±0.9 preoperatively to 0.9±1.1 at a 5-year follow-up visit (p<0.001). [10]
Reported complications of ELT were rare and included short-term micro hyphema, which was clinically insignificant.
Multiple studies have investigated the role of combining ELT with phacoemulsification. In a study by Töteberg-Harms and colleagues[11] including 28 eyes with a mean follow-up duration of 12 months, the combination of ELT with phacoemulsification resulted in a 34.7% reduction in the mean IOP from 25.33±2.85 mmHg at baseline to 16.54±4.95 mmHg at 1-year follow up (p<0.001), and a 0.79 mean reduction in medication usage from 2.25±1.26 preoperatively to 1.46±1.38 (p=0.017) at 1-year follow up. Another study by the same group included 64 eyes with a follow-up duration of 1 year. The eyes were classified into two groups according to baseline IOP: those with ≤21 mmHg preoperative IOP (study group) and those with >21 mmHg (control group). In the control group, the mean IOP decreased from 25.8±2.9 mmHg at baseline to 16.4±5.4 mmHg (p < 0.001) at the 1-year follow-up visit, and mean medication usage decreased from 2.2±1.4 at baseline to 1.6±1.5 at the 1-year follow-up visit (p<0.001). In contrast, in the study group, the mean IOP decreased from 16.5±2.9 mmHg at baseline to 14.6±3.7 mmHg at the 1-year follow-up visit (p < 0.001), and mean medication usage decreased from 2.5±1.0 at baseline to 1.4±1.3 at the 1-year follow-up visit (p<0.001).[12]
Another study by Moreno Valladares and colleagues [6] with a mean follow-up duration of 11.5±0.5 months, including 34 eyes, found a mean IOP reduction from 20.9±2.6 mmHg at baseline to 16.3±1.9 mmHg at the 1-year follow-up visit (p<0.0001). In addition, the number of glaucoma medications decreased from a mean of 1.7±0.7 at baseline to 0.3 ± 0.8 at the 1-year follow-up visit (p<0.001), with 81% of the eyes being medication-free.
Töteberg-Harms and colleagues [13] compared the long-term outcomes of phacoemulsification and trabeculectomy to combined phacoemulsification with ELT over a follow-up period of 4 years. The study revealed significant median IOP reduction, with trabeculectomy eyes showing a decrease in median IOP from 22.8 mmHg to 14.0 mmHg (p<0.001). On the other hand, the ELT eyes showed IOP reduction from 19 mmHg to 14 mmHg (p=0.002). In addition, medication reduction was significant in both groups.
Jozic and colleagues [14] assessed the effects of different surgical interventions, including phacoemulsification alone, ELT with phacoemulsification, and ab-interno trabeculotomy (aiT) using the trajectory device (NeoMedix, Tustin, USA) with phacoemulsification, in 245 eyes with cataract and glaucoma over a follow-up period of 1 year. In the phaco alone group, mean IOP was reduced from 16.7±3.8 at baseline to 15.2±3.1 mmHg (p=0.015) at the 1-year follow-up visit, while the mean number of glaucoma medications was reduced from 1.1±0.6 to 1.0±0.7; however, the difference was not statistically significant (p= 0.438) during the same period. In the combined ELT with phacoemulsification group, mean IOP was lowered from 17.8±4.3 mmHg at baseline to 13.2±2.3 mmHg (p=0.001) at the 1-year follow-up visit and a mean number of glaucoma medications was significantly reduced from 1.4±0.7 to 0.5±0.8 (p=0.001) during the same period. In the aiT with phacoemulsification group, mean IOP was lowered from 19.3±4.6 mmHg at baseline to 13.8±2.2 mmHg (p=0.001) at the 1-year follow-up visit, and the mean number of glaucoma medications was significantly reduced from 1.3±0.8 to 0.5±0.7 (p=0.001) during the same period.
Riesen and colleagues [7] studied 161 eyes with primary, secondary glaucoma, and ocular hypertension that underwent ELT combined with phacoemulsification over a follow-up period of 8 years. They found a significant reduction in mean IOP from 19.3±4.8 mmHg at baseline to 15.4±3.2 mmHg (p=0.004) at an 8-year follow-up visit. However, the mean number of glaucoma medications used did not change significantly from a mean of 2.3±1.2 medications at baseline to 2.1±1.2 medications (p=0.52) at the 8-year follow-up visit.
Table illustrating the important studies on ELT
| Study | Sample size | Age | Procedure | Follow-up duration | IOP reduction | Antiglaucoma medications reduction |
|---|---|---|---|---|---|---|
| Vogel (1996)[8] | 6 eyes with POAG | -- | ELT | 5 months | 11 mmHg reduction in 4 eyes | Not studied |
| Vogel (1997)[9] | 27 eyes with POAG and 8 eyes with low-tension glaucoma | -- | ELT | 7 months | In POAG eyes: median of 7 mmHg reduction (range 10.5 to 1.5 mmHg) in 22 of 27 eyes with chronic glaucoma.
Low tension glaucoma eyes: median of 5 mmHg reduction (range 10 to 0.5 mmHg) in all 8 eyes |
In POAG eyes: 12 eyes medication continued lower doses.
In low tension glaucoma eyes: 5 eyes continued lower doses |
| Stodtmeister (2011)[4] | 166 eyes with POAG and secondary glaucoma | 70±13 years | ELT | 1 year | Mean reduction from 26.4±6.2 mmHg to 16.9±4.7 mmHg at the end of follow up (p<0.00001) | No medication changes before surgery |
| Stodtmeister (2013)[10] | 46 eyes; 35 eyes with Ocular hypertension, 7 eyes with Secondary glaucoma, 2 eyes with PEX glaucoma | 64 ± 18 years | ELT | 5 years | Mean reduction from 25.5±6.3 mmHg to 15.9±3.0 mmHg at the end of follow up (p<0.001) | decreased from mean of 1.9± 0.9 pre-op to 0.9±1.1 (p<0.001) |
| Töteberg-Harms (2011)[11] | 28 eyes; POAG in 9 eyes; PEX glaucoma in 15 eyes; ocular hypertension in 3 eyes, and 1 post-traumatic secondary glaucoma | 74.33±11.81 years | ELT + Phacoemulsification | 12 months | Mean reduction from 25.33±2.85 to 16.54±4.95 (p<0.001) at the end of follow up | Reduced from mean of 2.25±1.26 to 1.46±1.38 (p=0.017) |
| Töteberg-Harms (2013)[12] | 64 eyes with POAG or ocular hypertension divided into:
Study group: preoperative IOP ≤21 mmHg and Control group: preoperative IOP >21 mmHg |
76.5 ± 9.4 years | ELT + phacoemulsification (in both study and control groups) | 12 months | Study Group: Mean reduction from 16.5±2.9 mmHg to 14.6±3.7 mmHg (p < 0.001) at the end of follow up
Control Group: Mean reduction from 25.8±2.9 mmHg to 16.4±5.4 mmHg (p < 0.001) at the end of follow up |
Study Group: reduced from mean 2.5±1.0 to 1.4 ±1.3 (p < 0.001)
control group: reduced from mean of 2.2±1.4 to 1.6±1.5 (p < 0.001) |
| Töteberg-Harms (2017)[13] | 113 eyes with cataracts and glaucoma | 76.1 ± 8.6 years | Phacoemulsification and trabeculectomy in 62 eyes and phacoemulsification + ELT in 51 eyes | 4 years | Median reduction from 22.8 mmHg to 14.0 mmHg (p< 0.001) in trabeculectomy eyes and from 19 mmHg to 14 mmHg in ELT eyes at the end of follow up (p=0.002) | Reduced from a median of 2 drugs to 0 in trabeculectomy eyes (p< 0.001) and from a median of 2 drugs to one drug in ELT eyes (p=0.002) |
| Jozic (2020)[14] | 245 eyes with cataracts and glaucoma | 74.8 years | Phacoemulsification alone only in 38 eyes, ELT + phacoemulsification in 105 eyes, and aiT + phacoemulsification in 102 eyes | 1 year | The mean reduction in phacoemulsification alone: from 16.7±3.8 mmHg to 15.2±3.1 mmHg (p=0.015) at the end of follow up
, ELT + phacoemulsification: from 17.8±4.3 mmHg to 13.2±2.3 mmHg (p=0.001) at the end of follow up , and aiT + phacoemulsification: from 19.3±4.6 mmHg to 13.8±2.2 mmHg at the end of follow up (p=0.001) |
Reduced in Phacoemulsification alone only eyes from mean of 1.1±0.6 to 1.0±0.7 (p=0.438), in ELT + phacoemulsification from a mean of 1.4±0.7 to 0.5± 0.8 (p=0.001), and in aiT + phacoemulsification from a mean of 1.3±0.8 to 0.5±0.7 (p<0.001) |
| Moreno Valladares (2021)[6] | 34 eyes with cataract and POAG | 73.1 ±8.8 years | ELT + Phacoemulsification | 11.5 ± 0.5 months | Mean reduction from 20.9±2.6 mmHg to 16.3±1.9 mmHg (p< 0.0001) at the end of follow up | Reduced from the mean of 1.7 to 0.3 (p< 0.001) |
| Riesen (2022)[7] | 161 eyes with cataracts and primary glaucoma, secondary glaucoma, or ocular hypertension | 75.4 (±9.1) years | ELT + Phacoemulsification | 8 years | Mean reduction from 19.3±4.8 mmHg at to 15.4±3.2 mmHg, (p=0.0040) at the end of follow up | Reduced from mean of 2.3±1.2 to 2.1±1.2 (p=0.52) |
Technique [1][6][7]
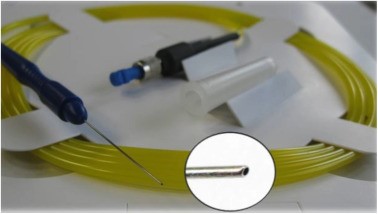
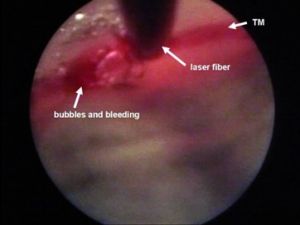
ELT is done with a short-pulsed (20 Hz, 60–120 ns) 308 nm xenon chloride (XeCl) excimer laser. The ELT “Fido” is a single use fiberoptic probe having a metallic angled tip designed to maximize contact with the TM. (Figure 1) After making a corneal paracentesis wound to introduce the laser probe into the anterior chamber, the tip is approached under gonioscopic guidance until it contacts the TM to transfer enough sub-threshold laser energy to the target tissue. Ten micro perforations (ELT channels) are created into the TM spread over a 90° area. The diameter of each ELT channel is about 0.2 mm. (Figure 2) Following laser application, bubble formation and a small amount of retrograde bleeding are seen, indicating that the TM and the inner wall of Schlemm's canal have been penetrated. After the surgery, the viscoelastic is removed from the anterior chamber, and the paracentesis wound is closed by stromal hydration.
Femtosecond Laser Image-Guided High-precision Trabeculotomy
Idea
By delivering tightly focused femtosecond laser pulses through the cornea and into the iridocorneal angle, the ViaLase® Laser technology (ViaLase Inc., Aliso Viejo, CA) optical scanning technology allows for precise photo disruption of the TM. Femtosecond Laser Image-Guided High-precision Trabeculotomy (FLIGHT) is intended to build a conduit that connects the anterior chamber to the Schlemm's canal non-invasively and precisely, increasing aqueous humor drainage.[2] [15] [16]
In perfused human cadaver eyes, FLIGHT has recently been shown to lower IOP with minimal or no collateral tissue injury, as determined by spectral-domain optical coherence tomography (SD-OCT) and transmission microscopy. [15][16]
Effectiveness and safety
Mikula and colleagues initially investigated the feasibility of the FLIGHT by delivering the femtosecond laser directly through the TM in a perfused human cadaver model. They compared FLIGHT (6 eyes) to sham treatment (6 eyes). In the FLIGHT group, they reported a significant reduction in the mean IOP from 7.1±2.9 mmHg at baseline to 5.3±1.62 mmHg after treatment (p<0.05). However, there was no significant change in the sham group (p=0.28). [16]
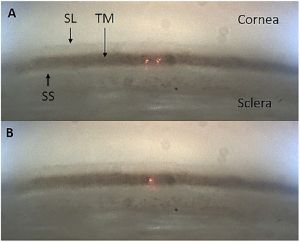
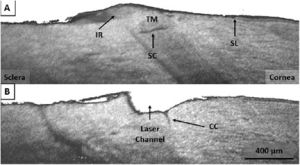
Another study from the same group investigated delivering tightly focused femtosecond laser pulses through the cornea (i.e., in a clinically relevant manner) and into the iridocorneal angle of the perfused anterior segment models and not directly through the TM as in the previous study (Figures 3, 4). They reported a significant reduction in the mean IOP from 5.06±1.4 mmHg at baseline to 4.04±1.6 mmHg in the FLIGHT group (6 eyes); however, there was no significant difference in the sham group’s mean IOP (7 eyes). [15]
In a prospective non-randomized pilot study, Nagy and colleagues [2] investigated the use of FLIGHT for glaucoma treatment in 18 eyes of 11 open-angle glaucoma patients aged >35. They excluded those with prior glaucoma procedures, including selective laser trabeculoplasty, and eyes with any ocular diseases that would impair TM visibility.
The authors did not report any serious adverse events, such as vision loss, corneal edema, hypotony, hyphema, peripheral anterior synechiae, or IOP spikes at any point during the study duration. Only transient conjunctival hemorrhage occurred in 16.7% (3 eyes), related to the coupling lens suction ring. One eye with pre-existing epiretinal membrane developed cystoid macular edema, which was resolved by topical Nepafenac. In addition, the authors reported 2 eyes lost more than 2 Snellen's lines during the follow-up period. However, the vision loss was related to glaucoma progression in one eye and cataract formation in the second eye and was not related to the laser treatment itself.[2]
They observed that after two years of follow-up, the mean IOP decreased from 22.3 ±5.5 mmHg preoperatively to 14.5±2.6 mmHg (p=0.00005). An average of 2.2±1.1 glaucoma medications preoperatively decreased to 2.0±1.2 at the 2-year postoperative follow-up visit (p=0.22). About 82% of eyes had an IOP reduction of ≥20% compared to baseline. There was no statistically significant difference in the visual field mean deviation at 24 months postoperatively compared to baseline. Gonioscopically and using anterior-segment OCT, well-defined channels were visible at 24 months.[2]
Technique [2]
Before placing the portable gonio-camera on the eye, gonio gel was placed on the cornea's surface. Then, the portable gonio-camera was used to check the iridocorneal angle and choose the site for treatment. The gonio-camera provides a real-time, high-resolution video of the angle. Next, using a suction ring attached to the coupling lens, the coupling lens was fitted onto the study eye and fixed in place with vacuum suction. The coupling lens cone was then docked with the laser system using a motorized x-y-z axis gantry and joystick. The investigator then used the system's real-time gonioscopic video to locate the TM before positioning the aiming lasers using the touchscreen interface. By turning the system's focusing knob, the aiming lasers overlapped onto the TM. The OCT was used to measure the depth of the surface of the TM with micron-level accuracy. After the laser has been adequately aimed, the user activates the preprogrammed laser therapy using a footswitch. Suction was released between the eye and the laser after the laser treatment was complete.
Conclusion
The ELT approach is safe and effective whether employed alone or in conjunction with phacoemulsification, with current evidence from several studies showing the long-term efficacy in reducing the IOP for at least two years. On the other hand, FLIGHT's initial safety profile is promising, and its potential to lower IOP is encouraging. However, the results are preliminary and further research is necessary.
References
- ↑ Jump up to: 1.0 1.1 Nguyen A, Simon B, Doan R, et al. Advances in Excimer Laser Trabeculostomy within the Landscape of Minimally-Invasive Glaucoma Surgery. J Clin Med. 2022;11(12).
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 Nagy ZZ, Kranitz K, Ahmed IIK, De Francesco T, Mikula E, Juhasz T. First-in-Human Safety Study of Femtosecond Laser Image Guided Trabeculotomy for Glaucoma Treatment: 24-month Outcomes. Ophthalmol Sci. Published online May 28, 2023.
- ↑ Babighian S, Caretti L, Tavolato M, Cian R, Galan A. Excimer laser trabeculotomy vs 180 degrees selective laser trabeculoplasty in primary open-angle glaucoma. A 2-year randomized, controlled trial. Eye (Lond). 2010;24(4):632-638.
- ↑ Jump up to: 4.0 4.1 4.2 Stodtmeister RP, Kleineberg L, Berlin MS, Pillunat LE, Giers U. Excimer Laser Trabeculostomy: One Year Post-OP Efficacy in 166 Eyes. Invest Ophthalmol Vis Sci. 2011;52(14):2630
- ↑ Babighian S, Rapizzi E, Galan A. Efficacy and safety of ab interno excimer laser trabeculotomy in primary open-angle glaucoma: two years of follow-up. Ophthalmol J Int d’ophtalmologie Int J Ophthalmol Zeitschrift fur Augenheilkd. 2006;220(5):285-290.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 Moreno-Valladares A, Puerto Amorós N, Mendez Llatas M, Pazos-López M, Ahmed IIK. Combined excimer laser trabeculostomy and phacoemulsification: One year follow-up real world data of a laser-based MIGS. Arch Soc Esp Oftalmol. 2021;96(12):631-639
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Riesen M, Funk J, Töteberg-Harms M. Long-term treatment success and safety of combined phacoemulsification plus excimer laser trabeculostomy: an 8-year follow-up study. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch fur Klin und Exp Ophthalmol. 2022;260(5):1611-1621.
- ↑ Jump up to: 8.0 8.1 Vogel M, Lauritzen K, Quentin CD. [Targetted ablation of the trabecular meshwork with excimer laser in primary open-angle glaucoma]. Ophthalmologe. 1996;93(5):565-568.
- ↑ Jump up to: 9.0 9.1 Vogel M, Lauritzen K. [Selective excimer laser ablation of the trabecular meshwork. Clinical results]. Ophthalmologe. 1997;94(9):665-667.
- ↑ Jump up to: 10.0 10.1 Stodtmeister R, Kleineberg L, Berlin M, Pillunat L, Giers U. Excimer Laser Trabeculostomy: Five Year Post-OP Observations. Invest Ophthalmol Vis Sci. 2013;54(15):2141.
- ↑ Jump up to: 11.0 11.1 Töteberg-Harms M, Ciechanowski PP, Hirn C, Funk J. [One-year results after combined cataract surgery and excimer laser trabeculotomy for elevated intraocular pressure]. Ophthalmologe. 2011;108(8):733-738.
- ↑ Jump up to: 12.0 12.1 Töteberg-Harms M, Hanson JV, Funk J. Cataract surgery combined with excimer laser trabeculotomy to lower intraocular pressure: effectiveness dependent on preoperative IOP. BMC Ophthalmol. 2013;13:24.
- ↑ Jump up to: 13.0 13.1 Töteberg-Harms M, Wachtl J, Schweier C, Funk J, Kniestedt C. Long-term efficacy of combined phacoemulsification plus trabeculectomy versus phacoemulsification plus excimer laser trabeculotomy. Klin Monbl Augenheilkd. 2017;234(4):457-463.
- ↑ Jump up to: 14.0 14.1 Jozic L, Magner J, Funk J, Töteberg-Harms M. Success of combined cataract extraction plus excimer laser trabeculotomy exceeds that of combined ab interno trabeculectomy with the trabectome or cataract extraction alone. Int Ophthalmol. 2020;40(3):529-537.
- ↑ Jump up to: 15.0 15.1 15.2 Mikula ER, Raksi F, Ahmed II, et al. Femtosecond Laser Trabeculotomy in Perfused Human Cadaver Anterior Segments: A Novel, Noninvasive Approach to Glaucoma Treatment. Transl Vis Sci Technol. 2022;11(3):28.
- ↑ Jump up to: 16.0 16.1 16.2 Mikula E, Holland G, Bradford S, et al. Intraocular Pressure Reduction by Femtosecond Laser Created Trabecular Channels in Perfused Human Anterior Segments. Transl Vis Sci Technol. 2021;10(9):22.

