Knobloch Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
First described by Knobloch and Layer in 1971, Knobloch syndrome is a rare recessively inherited disorder characterized by high myopia, vitreoretinal degeneration and occipital skull defects, with a high degree of phenotypic variability.[2][3][4] Mutations in the COL18A1 gene are causative for Knobloch syndrome, leading to the production of abnormal type XVIII collagen proteins.[5][6][7][8]
Disease Entity
Epidemiology
Knobloch syndrome is a rare disorder and since its original report, at least 90 cases from 48 families have been described.[3][9] Knobloch syndrome has not been reported to be correlated to any specific ethnic group. Cases have been reported in many ethnic groups including Brazilian, North American, Algerian, Hungarian, El Salvadoran, Saudi Arabian, and Chinese.[3][10]
Pathophysiology
Knobloch syndrome is caused by an autosomal recessive mutation in the COL18A1 gene located on the long arm of chromosome 21 (chr21q22.3) leading to changes in or a lack of type XVIII collagen in epithelial basement membranes (BM).[3][6][9] Type XVIII collagen serves multiple functions in neurological and ocular development including angiogenesis, BM maintenance, and Wnt/β signaling.[5] Type XVIII collagen is found in many ocular structures including Bruch's membrane, the lens capsule, the BM of the iris, the aqueous humor, vitreous, and retina.[11] The COL18A1 gene includes 43 exons, has three distinct isoforms in humans, and at least 20 polymorphic changes have been identified in Knobloch syndrome.[7][12] Additional research suggests the ADAMTS18 gene defines a novel pathogenic locus in patients with Knobloch syndrome.[12]
Risk Factors
Patterns of inheritance of Knobloch syndrome in an inbred Brazilian family confirmed an autosomal recessive inheritance pattern and consanguinity as a risk factor for the disease.[9][13][14]
Diagnosis
History and symptoms
Ocular abnormalities and occipital skull defects are the defining characteristics of Knobloch syndrome. Ocular abnormalities are present in all patients and often onset before one year of age. Symptoms may include blurry vision, nystagmus, and ocular misalignment.[2][8][15][16][17] Occipital skull defects may or may not be present. Occipital abnormalities range from scalp defects to encephalocele.[2][9]
Neurological examination and findings
Occipital skull defects with or without encephalocele are the characteristic findings in Knobloch syndrome. Neuroradiologic imaging has demonstrated additional central nervous system (CNS) findings in a minority of cases including polymicrogyria, subependymal nodules, and cerebellar vermis atrophy. Delay in cognitive development is frequently observed in the setting of CNS lesions, however, it is not considered a hallmark feature of Knobloch syndrome.[8][9]
Other systemic signs
Other signs present in a minority of cases include hypoplasia of the right lung with an anomalous pulmonary return, cardiac dextroversion, flat nasal bridge, midface hypoplasia, bilateral epicanthic folds, generalized hyperextensibility of the joints, unilateral duplicated renal collecting system, and unusual palmar creases.[7][14]
Ophthalmologic findings
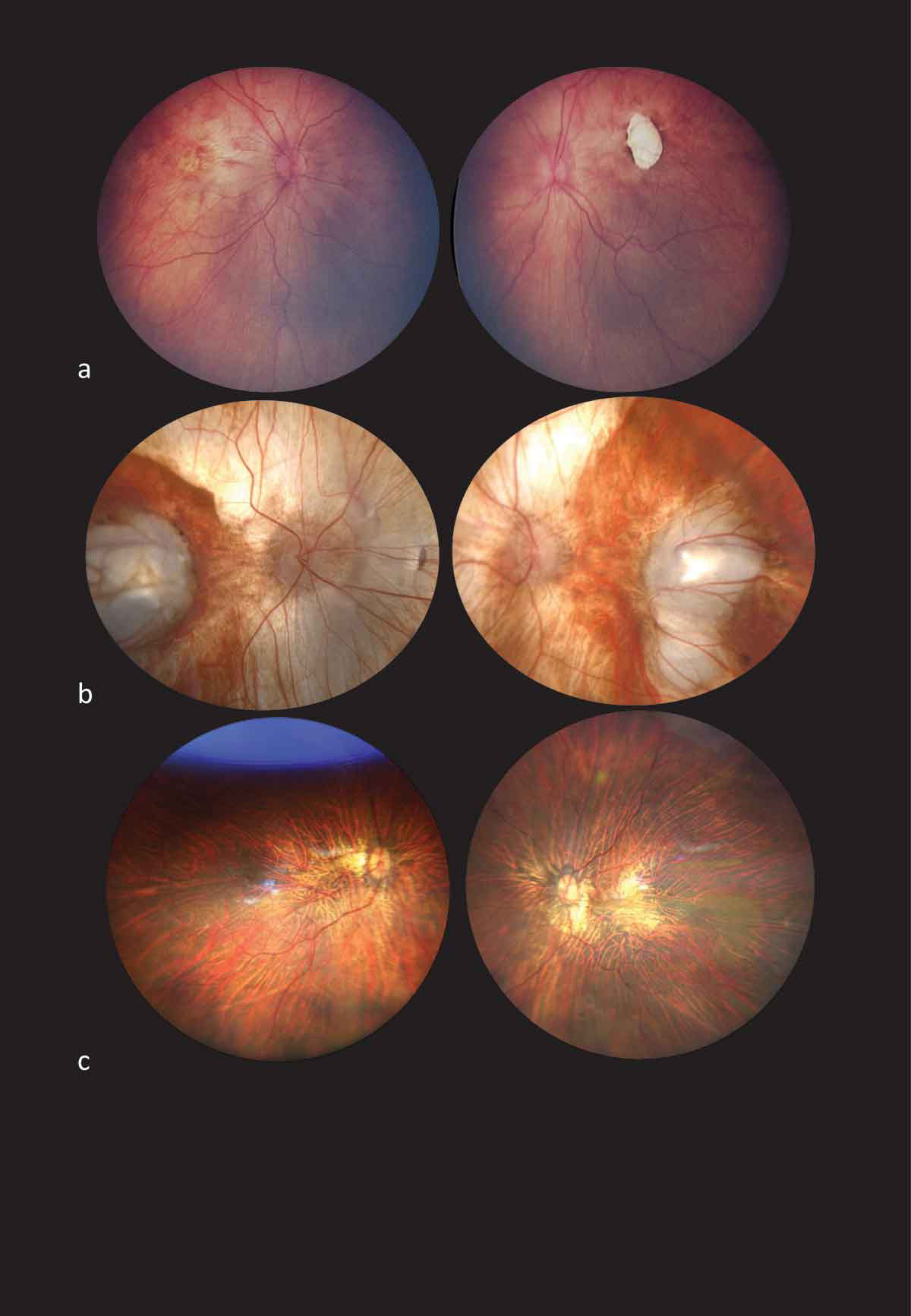
High myopia (usually with an absolute value of more than 10 diopters) and vitreoretinal degeneration (often progressing to retinal detachment) are the hallmark ophthalmic findings in Knobloch syndrome.[2][3][10] Ocular abnormalities may also include lens subluxation, retinal detachments, cataracts, strabismus and macular abnormalities.[2][7][9][14] Khan et al. describes the distinct fundoscopic findings in patients with Knobloch syndrome as diffuse severe chorioretinal atrophic changes with prominent choroidal vessel show, colobomas, macular atrophic lesions with or without a punched out appearance, macular hypoplasia and white fibrillar vitreous condensations.[10] Additionally, smooth irides and posterior perinuclear lens opacity are recurrent features in patients with Knobloch syndrome.[10] Knobloch syndrome demonstrates clinical heterogeneity and may also present with retinitis pigmentosa-like features.[3][7][13][18] Recent studies indicate cone and rod dysfunction can be observed on electroretinogram (ERG).[2]
The ocular features of Knobloch syndrome include:[19][20]
- Congenital cataracts,
- Band-shaped keratopathy,
- Nystagmus,
- Poor pupillary dilation,
- Persistent pupillary membrane,
- Absence of iris crypts,
- Transillumination defects of iris,
- Glaucoma and glaucomatous optic neuropathy,
- Acute angle closure glaucoma
- Pigment dispersion syndrome,
- Syneresis,
- Persistent fetal vasculature,
- Attenuated retinal vessels,
- Macular or generalized chorioretinal atrophy,
- Macular hypoplasia,
- Pallor of the optic disc, and
- Irregular white dots at the vitreoretinal interface.
Clinical diagnosis
Diagnosis of Knobloch syndrome is based on the presence of the classical triad of high myopia, vitreoretinal degeneration, and occipital findings (ranging from scalp defect to encephalocele) with infantile-onset. Khan et al. suggest smooth irides, ectopia lentis, and characteristic vitreoretinal degeneration are pathognomonic for Knobloch syndrome.[10][21] Knobloch syndrome can occur without clinical evidence of occipital defects and can be diagnosed by eye examination alone.[10] Optical coherence tomography of the macula may show the absence of the foveal pit, loss of outer retinal structures, severe retinal thinning, and macular pseudocoloboma.[19] Fundus autofluorescence highlights the areas of RPE damage more clearly.
A diagnosis of Knobloch syndrome can be confirmed by the presence of COL18A1 mutations.[3][9] Immunohistochemical assessment of type XVIII collagen and endostatin measurements through ELISA have also been employed to diagnosis Knobloch syndrome.[6]
Differential diagnosis
Differential diagnoses may include other forms of congenital vitreoretinopathies, although each has unique defining features. In Stickler syndrome, there is often radial perivascular retinal degeneration, a lack of macular atrophic lesions, good best-corrected visual acuity and midface hypoplasia.[22] Autosomal dominant vitreoretinochoroidopathy shows peripheral and delineated severe retinal degeneration and is often associated with anterior segment dysgenesis.[23]
Management
General treatment
Treatment for Knobloch syndrome is supportive and aimed at treating the symptoms in each individual including surgical repair of both ocular and occipital skull defects.[8]
Ophthalmologic treatment
Lens extraction may be indicated for ectopia lentis or lenticular opacification, but there is a substantially increased risk of capsular rupture (likely due to modified collagen affecting the integrity of the lens capsule and zonules).[24] Due to the high risk of retinal detachment, prophylactic scleral buckle placement and/or laser or cryo-retinopexy are sometimes offered.[25] [8]
Genetic planning
Knobloch syndrome is an autosomal recessive condition that requires a mutation in both copies of the COL18A1 gene. Parents of an affected individual carrying a single mutated copy of this gene do not present with the clinical syndrome but confer a risk of 25% for Knobloch syndrome to each offspring.[2][8][1]
Prognosis
Ocular abnormalities in Knobloch syndrome are severe, progressive, and irreversible, usually leading to bilateral blindness.[7][14] Incidence of retinal detachment is near universal even with surgical intervention and prophylactic cryotherapy. Retinal detachments tend to occur at the end of the first decade of life or later.[8]
References
- ↑ Jump up to: 1.0 1.1 Balikova I, Sanak NS, Fanny D, et al. Three cases of molecularly confirmed Knobloch syndrome. Ophthalmic Genetics 2020;41(1):83-87 doi: 10.1080/13816810.2020.1737948
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Hull S, Arno G, Ku CA, et al. Molecular and Clinical Findings in Patients With Knobloch Syndrome. JAMA Ophthalmology 2016;134(7):753-62 doi: 10.1001/jamaophthalmol.2016.1073
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Zhang LS, Li HB, Zeng J, Yang Y, Ding C. Knobloch syndrome caused by homozygous frameshift mutation of the COL18A1 gene in a Chinese pedigree. Int J Ophthalmol 2018;11(6):918-22 doi: 10.18240/ijo.2018.06.04
- ↑ Knobloch WH, Layer JM. Retinal Detachment and Encephalocele. JPOS 1971;8(3):181-84 doi: doi:10.3928/0191-3913-19710801-11
- ↑ Jump up to: 5.0 5.1 Seppinen L, Pihlajaniemi T. The multiple functions of collagen XVIII in development and disease. Matrix Biology 2011;30(2):83-92 doi: https://doi.org/10.1016/j.matbio.2010.11.001
- ↑ Jump up to: 6.0 6.1 6.2 Suzuki O, Kague E, Bagatini K, et al. Novel pathogenic mutations and skin biopsy analysis in Knobloch syndrome. Mol Vis 2009;15:801-09
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 Suzuki OT, Sertié AL, Der Kaloustian VM, et al. Molecular analysis of collagen XVIII reveals novel mutations, presence of a third isoform, and possible genetic heterogeneity in Knobloch syndrome. Am J Hum Genet 2002;71(6):1320-9 doi: 10.1086/344695
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 8.6 White RJ, Wang Y, Tang P, Montezuma SR. Knobloch syndrome associated with Polymicrogyria and early onset of retinal detachment: two case reports. BMC Ophthalmology 2017;17(1):214 doi: 10.1186/s12886-017-0615-z
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Caglayan AO, Baranoski JF, Aktar F, et al. Brain malformations associated with Knobloch syndrome--review of literature, expanding clinical spectrum, and identification of novel mutations. Pediatr Neurol 2014;51(6):806-13.e8 doi: 10.1016/j.pediatrneurol.2014.08.025
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 10.5 Khan AO, Aldahmesh MA, Mohamed JY, Al-Mesfer S, Alkuraya FS. The distinct ophthalmic phenotype of Knobloch syndrome in children. Br J Ophthalmol 2012;96(6):890-5 doi: 10.1136/bjophthalmol-2011-301396
- ↑ Heljasvaara R, Aikio M, Ruotsalainen H, Pihlajaniemi T. Collagen XVIII in tissue homeostasis and dysregulation - Lessons learned from model organisms and human patients. Matrix Biol 2017;57-58:55-75 doi: 10.1016/j.matbio.2016.10.002
- ↑ Jump up to: 12.0 12.1 Aldahmesh MA, Khan AO, Mohamed JY, et al. Identification of ADAMTS18 as a gene mutated in Knobloch syndrome. Journal of Medical Genetics 2011;48(9):597-601 doi: 10.1136/jmedgenet-2011-100306
- ↑ Jump up to: 13.0 13.1 Passos-Bueno MR, Marie SK, Monteiro M, et al. Knobloch syndrome in a large Brazilian consanguineous family: confirmation of autosomal recessive inheritance. Am J Med Genet 1994;52(2):170-3 doi: 10.1002/ajmg.1320520209
- ↑ Jump up to: 14.0 14.1 14.2 14.3 Sertié AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of the retinal structure and in neural tube closure (Knobloch syndrome). Human Molecular Genetics 2000;9(13):2051-58 doi: 10.1093/hmg/9.13.2051
- ↑ Li S, Wang Y, Sun L, et al. Knobloch Syndrome Associated with Novel COL18A1 Variants in Chinese Population. Genes 2021;12(10):1512
- ↑ Villafuerte R, Villanueva-Mendoza C, García-Albisua AM, Cortés-González V. Knobloch syndrome associated with terminal transverse defect. Investigative Ophthalmology & Visual Science 2015;56(7):5991-91
- ↑ Williams TA, Kirkby GR, Williams D, Ainsworth JR. A Phenotypic Variant of Knobloch Syndrome. Ophthalmic Genetics 2008;29(2):85-86 doi: 10.1080/13816810701850041
- ↑ Haghighi A, Tiwari A, Piri N, et al. Homozygosity mapping and whole exome sequencing reveal a novel homozygous COL18A1 mutation causing Knobloch syndrome. PLoS One 2014;9(11):e112747 doi: 10.1371/journal.pone.0112747
- ↑ Jump up to: 19.0 19.1 OMIM # 267750 KNOBLOCH SYNDROME 1; KNO1 [Internet]. OMIM [cited 2022 Sep 13];Available from: https://www.omim.org/entry/267750
- ↑ Wawrzynski J, Than J, Gillam M, Foster PJ. Acute Angle Closure in Knobloch Syndrome. J Glaucoma. 2021 May 1;30(5):e265-e268.
- ↑ Kliemann SE, Waetge RTL, Suzuki OT, Passos-Bueno MR, Rosemberg S. Evidence of neuronal migration disorders in Knobloch syndrome: Clinical and molecular analysis of two novel families. American Journal of Medical Genetics Part A 2003;119A(1):15-19 doi: https://doi.org/10.1002/ajmg.a.20070
- ↑ Donoso LA, Edwards AO, Frost AT, et al. Clinical variability of Stickler syndrome: role of exon 2 of the collagen COL2A1 gene. Surv Ophthalmol 2003;48(2):191-203 doi: 10.1016/s0039-6257(02)00460-5
- ↑ Yardley J, Leroy BP, Hart-Holden N, et al. Mutations of VMD2 Splicing Regulators Cause Nanophthalmos and Autosomal Dominant Vitreoretinochoroidopathy (ADVIRC). Investigative Ophthalmology & Visual Science 2004;45(10):3683-89 doi: 10.1167/iovs.04-0550
- ↑ Bongiovanni CS, Serra Ferreira CC, Silvério Rodrigues AP, Fortes Filho JB, Tartarella MB. Cataract surgery in Knobloch syndrome: a case report. Clin Ophthalmol. 2011; 5: 735–737. doi: 10.2147/OPTH.S18989
- ↑ Moysidis SN, Aziz HA, Rachitskaya AV, Berrocal AM. Prophylactic scleral buckle implantation in Knobloch syndrome. J Pediatr Ophthalmol Strabismus. 2014;51:e40–3.