Intra-arterial Chemotherapy for Retinoblastoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Intra-arterial (IA) chemotherapy for the treatment of intraocular retinoblastoma, also referred to as superselective intra-arterial chemotherapy and chemosurgery, is a new treatment regimen that is gaining popularity. The procedure is being performed in more than 31 countries worldwide [1] [2]. More than twenty peer-reviewed publications have been published on this promising therapy, and the November 2011 retinoblastoma themed issue of Archives of Ophthalmology featured several studies on intra-arterial chemotherapy. The purpose of this article is to provide a comprehensive review of this treatment modality for the practicing ophthalmologist.
History
Intra-arterial chemotherapy for the treatment of intraocular retinoblastoma was first performed by Algernon B. Reese with direct internal carotid artery (ICA) injection of the alkylating agent triethylene melamine (TEM) in 1954 [3]. Other investigators including Kiribuchi in Japan in 1968 experimented with local delivery of drug to the eye comparing ocular tissue concentration of mitomycin in dogs when treated via the common carotid artery and the external ophthalmic artery by way of the infraorbital artery [4]. The principal motivating factor was to deliver increased local concentration of drug to eyes without excessive systemic toxicity which is not possible with systemic administration.
The idea of local delivery of chemotherapy for retinoblastoma was later revisited by Yamane & Kaneko in 2004 when they described the technique of ‘selective ophthalmic artery infusion’ (SOAI) where a micro-balloon catheter is positioned by a transfemoral artery approach at the cervical segment of the internal carotid artery just distal to the orifice for the ophthalmic artery. At this point, the balloon catheter is inflated, and chemotherapy is injected with flow thereby directed into the ophthalmic artery [5]. The authors of this study noted there are several small, but nevertheless important, branches proximal to the origin of the ophthalmic artery (i.e. cavernous branches of the ICA) into which infused chemotherapy could flow, and concluded, “Therefore, strictly speaking, our infusion method is not truly selective.” [5]
The Japanese technique of ‘selective ophthalmic artery infusion’ was further developed into ‘direct intra-arterial (ophthalmic artery) infusion’ under the pioneering work of Abramson and Gobin in New York, NY at Memorial Sloan-Kettering Cancer Center and New York-Presbyterian Hospital/Weill Cornell Medical Center under an institutional review board approved-protocol that began in May 2006 [6]. Abramson’s initial report on the technique was seminal as it was the first truly selective delivery by direct catheterization of the ophthalmic artery which could be performed reliably, quickly, efficiently and safely in young children with intraocular retinoblastoma.
Indications
It is important to note intra-arterial treatment is intended for intraocular retinoblastoma and not for disease with extra-ocular involvement which requires consultation with a pediatric oncologist and is beyond the scope of this article.
Studies have demonstrated that the direct ophthalmic artery catheterization technique can be used successfully as primary therapy (i.e. naïve eyes that have received no previous treatment) [6][7] [8] [9] [10], especially with advanced disease (Reese Ellsworth [RE] Group IV/V, International Classification of Retinoblastoma [ICRB] Group D/E) [8][11], in bilateral cases (treating both eyes, ‘tandem therapy’) [12] and in eyes that have previously failed conventional management including external beam radiation and systemic chemotherapy [6][9][10][11].
Although many different treatment strategies can be used to treat intraocular retinoblastoma, a clinical dilemma exists when approaching the treatment of eyes with advanced intraocular disease (RE Group IV/V or ICRB D/E) [1]. Prior to IAC, the most common primary modality for intraocular retinoblastoma was systemic chemotherapy followed by local therapy also known as ‘chemoreduction’. External beam radiotherapy was used prior to the era of chemoreduction as primary therapy but is now used mainly for salvage of eyes with recurrences (see section below Clinic Outcomes Compared to External Beam Radiation and Systemic Chemotherapy). Only approximately 20-25% of RE V eyes avoided enucleation with external beam irradiation as described by Reese et al. [13], and the best results for advanced RE V eyes treated with chemoreduction published by Shields et al. report that 47% required EBR and 53% required enucleation at 5 years [14]. This highlights the inadequacy of these treatment modalities for eyes with advanced disease.
Seeding of any type (vitreous or subretinal) is a poor prognostic indicator; however, a recent study with 2 year results shows that unlike EBR or chemoreduction, intra-arterial chemotherapy can more often prevent enucleation in naïve eyes with intraocular retinoblastoma, especially with subretinal seeds [15].
Initial reports also indicate that eyes with advanced intraocular disease presenting with retinal detachment treated with intra-arterial therapy appear to have a better prognosis than eyes without retinal detachment even if subretinal seeds are present [2]. Moreover, intra-arterial treatment can resolve partial retinal detachment in 100% of cases and total retinal detachment in 43-75% [2][16] [17].
Intra-arterial agents used to treat retinoblastoma
The three principal chemotherapeutics used intra-arterially are melphalan, topotecan and carboplatin.
The alkylating agent melphalan (trade name Alkeran®) has been the most extensively used intra-arterial chemotherapeutic agent for the treatment of intraocular retinoblastoma [11]. The rationale for the use of this drug is based on in vitro studies with human cultured retinoblastoma cells using clonogenic assays which showed, compared to other commonly used chemotherapeutic drugs, melphalan had the greatest effect on retinoblastoma cells [18]. Systemic intravenous usage of melphalan is limited by severe myelosuppresion, but when administered intra-arterially is well tolerated at doses less than 0.5 mg/kg [11]. Most commonly, doses of 2.5-7.5 mg are administered intra-arterially to a single affected eye [1], though there is variation in dosing at different centers[9][10][19] [20] [21].
Topotecan (trade name Hycamtin®), a topoisomerase inhibitor, has been increasingly used intra-arterially for aggressive cases not responsive to single-agent melphalan. Rodent studies showed topotecan with carboplatin compared to vincristine, carboplatin and etoposide most effectively halted retinoblastoma progression in an animal model [22]. Several studies have investigated the use of periocular topotecan in the clinical setting [23] [24]. Lending further support for the use of this agent intra-arterially, is a recent study conducted in a porcine model that compared the vitreous and plasma pharmacokinectics of topotecan delivered intra-arterially via the ophthalmic artery versus periocular injection. Superselective ophthalmic artery infusion of topotecan resulted in a significantly higher vitreous concentration and exposure time with a trend toward lower systemic exposure when compared to periocular injection [23]. Most commonly, doses of 0.3-0.4 mg are administered intra-arterially to a single affected eye [1].
The alkylating agent carboplatin (trade name Paraplatin ®, Paraplatin-AQ®) has been used in three clinical contexts in New York. First, in cases where tumor has failed to respond to melphalan and/or topotecan it can be used intra-arterially as single-agent therapy [11]. Second, more commonly, it can be used in a simultaneous multi-agent triple therapy (melphalan + topotecan + carboplatin) in eyes that have failed single or double agent intra-arterial therapy or systemic intravenous chemotherapy [25]. Third, carboplatin may be used as an additional agent in tandem intra-arterial therapy for bilateral retinoblastoma when infusing melphalan to both eyes during a single treatment session; the dose of melphalan may need to be reduced when administered bilaterally to avoid myelosuppresion [11]. Most commonly doses of 15-30 mg are administered intra-arterially to a single affected eye [1], though there is variation in dosing and indication for use at other centers [9][10]
Gobin et al. described the dosing regimen of the above three chemotherapeutics in detail [11]. Drug dosage is determined by age, individual angioanatomy with body weight only taken into account for the youngest children receiving bilateral treatment. Extent of disease with particular attending to vitreous and subretinal seeding as well as clinical response to previous intra-arterial treatments also dictates the number and dose of agents used at subsequent IA sessions.
Preclinical studies have demonstrated that cardenolide glycosides such as digoxin have anti-tumor activity against retinoblastoma in vitro and in vivo [26]. A case report indicated oral administration of digoxin was unable to achieve sufficient intraocular levels to be therapeutic, however, there was a modest response with intra-arterial delivery of digoxin [26]. Methotrexate has been reported to be used twice intra-arterially, but ineffective at doses of 6 mg and 12 mg [11].
Procedure
Proper delivery of intra-arterial chemotherapy requires the cooperation of an integrated team including ocular oncologist, skilled neurointerventional radiologist, anesthesiologist, pediatric oncologist, pharmacist, nursing and other ancillary staff. A proper protocol and detailed plan should be in place before initiating this therapy. The procedure has been referred to as ‘chemosurgery’ as the precision, real-time decision making and other aspects are akin to a complex surgical procedure.
The protocol and procedure used in New York has been published [11] and will be briefly summarized here. The procedure is performed as an outpatient under general anesthesia with all patients requiring intubation. The common femoral artery on the intended side of treatment is accessed by the neurointerventional radiologist and the patient is then anticoagulated with intravenous heparin. A guide wire and microcatheter is then used to access the appropriate vascular supply to the eye with special attention to the angioanatomy and blood flow patterns in each patient.
Routes of delivery of chemotherapy
In the majority of cases the microcatheter is positioned at the ostium of the ophthalmic artery (OA) and a selective angiogram is performed with contrast to verify placement, angioanatomy and choroidal blush indicating proper flow to the eye.
In approximately 12.5% of all intra-arterial infusions the ophthalmic artery cannot be catheterized secondary to a variety of factors [27]. These factors include: small size of the OA due to young patient age, alternative vascular supply to the eye most commonly the middle meningeal artery (MMA) in 1-5% of the population [28] [29] [30], OA vasospasm during the procedure, and difficultly catheterizing the OA due to its steep take off from the ICA. When the OA cannot be accessed there are two other routes of treatment. First, the external carotid artery circulation is accessed to catheterize the orbital branch of the MMA, and a selective angiogram is performed to determine if this branch is well developed and may be used for delivery of chemotherapy. If the MMA route fails, the third option used by the New York group is temporary balloon occlusion of the ICA just distal to the origin of the ophthalmic artery, similar to the Japanese technique described by Yamane et al. [5], except Gobin et al. utilize a guide-wire directed instead of flow-guided catheter. Two other case reports have also investigated other routes of delivery of intra-arterial chemotherapy for the treatment of retinoblastoma [31] [32].
Infusion of chemotherapeutic
Chemotherapeutics are diluted in 30cc of normal saline and manually injected in a pulsatile fashion over 30 minutes to disrupt laminar flow and allow dispersion of drug to the supplied vascular territory [11]. When using the balloon-occlusion technique, alternating infusion (balloon inflation, 4 minutes) and reperfusion time (balloon deflation, 2 minutes) are employed to ensure adequate cerebral perfusion. With the balloon technique, drugs are each diluted in 6cc of normal saline and infused over the 4 minutes balloon inflation time in order to limit the total number of balloon infusion cycles to no more than three if multi-agent therapy is administered [11][27].
Conclusion of procedure
After completion of drug infusion, the catheter is removed and hemostasis of the femoral artery is achieved with manual pressure. Children are observed for 4-6 hours in a recovery unit and then discharged home.
Post-procedure management
A complete blood count with platelets is recommended for all patients 7-10 days after the intra-arterial procedure to monitor for myelosuppresion. Ophthalmic examinations should be performed every 3-4 weeks after treatment and include external examination, visual acuity testing, intraocular pressure measurement, pupil and motility evaluation and a complete fundus exam under anesthesia with large fundus drawings. Complete fundus examination, RetCam digital photography and B-scan ultrasonography are used as objective measures to monitor for decrease in tumor size, improvement of tumor seeding (either diminution or calcification), and resolution of retinal detachment to determine the effectiveness of individual treatment sessions.
To monitor for retinal toxicity, Abramson et. al perform electroretinogram (ERG) testing under photopic and scotopic conditions in accordance with modified International Society for Clinical Electrophysiology of Vision (ISCEV) standards as described in a previous publication [33]. The amplitude of the response to a 30-Hz flicker, measured in microvolts (mV), is used to monitor retinal function. Other groups have advocated for fluorescein angiography to monitor for retinal toxicity [19][34].
Clinical Outcomes
The largest clinical series on intra-arterial chemotherapy as a treatment for intraocular retinoblastoma have been conducted by Abramson et al. in New York. A number of reports from the New York group on their initial experiences with intra-arterial therapy [6][7][12][35]were published, and have been expanded upon with the largest series reported in 2011 [11].
Largest clinical series to date
Gobin et al. reported a nonrandomized prospective study of 95 eyes of 78 patients with unilateral or bilateral disease treated at a tertiary care ocular oncology center from 2006 to 2010 with selective catheterization of the ophthalmic artery with infusion of chemotherapy [11]. Stage at presentation included RE Vb – 73 eyes, RE Va –10 eyes, RE IV – 4 eyes, RE I-III – 8 eyes. 52 eyes (54.7%) were previously unsuccessfully treated with systemic chemotherapy or EBR. Catheterizations were successful in 98.5% (255 of 259 infusions). Eyes were treated with a mean number of 3.1 infusions (median 3, range 2-7). Chemotherapy most commonly delivered intra-arterially was melphalan with or without topotecan, and some patients also received carboplatin. 23% of eyes received no other additional treatments besides IA therapy. 57% of eyes received additional local therapy including local cryotherapy or laser ablation. Kaplan-Meier estimates of eye survival until enucleation or EBR therapy at 2 years was 70.0% for all eyes, 81.7% for eyes that received IA as a primary treatment and 58.4% for eyes that previously received IV systemic chemotherapy and/or EBR. For group V eyes, Kaplan-Meier estimates of ocular event-free survival at 2 years was 66.5% for all eyes, 80.5% for eyes that received IA as a primary treatment and 51.5% for eyes that previously received IV systemic chemotherapy and/or EBR. Median follow-up was 13 months (range 1-29 months). No children died, two children developed metastatic disease that is currently in remission [1] and no patient developed trilateral retinoblastoma. None of the RE I-IV eyes required enucleation, but 19 of 83 RE V eyes were enucleated.
The second largest study, also reported by Abramson et al., expanded on the 4 year experience report above by evaluating the effectiveness of IA treatment in advanced eyes with respect to vitreous and/or subretinal seeding [15]. The retrospective study evaluated 76 eyes of 67 patients with retinoblastoma and subretinal and/or vitreous seeding treated with IA chemotherapy (both naïve and previously treated eyes) in New York between 2006 and 2010. Stage at initial presentation included RE Vb – 68 eyes, RE Va – 8 eyes (ICRB Group C – 17 eyes, Group D – 49 eyes, Group E – 10 eyes). 43 eyes (56.5%) were previously treated with systemic chemotherapy or EBR, 4 eyes (5.2%) previously received local treatments including cryotherapy or laser and 29 eyes (38.1%) received no previous treatment and received IA chemotherapy as a primary treatment. The median follow-up for surviving eyes was 2.04 years (range 0.19-5.04). For naïve eyes, the 2 year Kaplan-Meier probability of ocular salvage was 83% for eyes with subretinal seeding only, 64% for eyes with vitreous seeding only, 80% for eyes with both. For eyes that had received previous treatment, the 2 year Kaplan-Meier probability of ocular salvage was 50% for eyes with subretinal seeding only, 76% for eyes with vitreous seeding only, 54% for eyes with both types of seeding. 20 enucleations were performed overall, 8 in eyes with only vitreous seeding, 5 in eyes with only subretinal seeding and 7 in eyes with both types of seeding.
More recent clinical series on IAC from MSKCC and Will's Eye Hospital appear to demonstrate increasing success rates of globe salvage. A publication from MSKCC in 2018 on a series of 452 eyes treated with IAC over an approximate 10 year period from 2006 and 2017, showed a 96 percent ocular survival rate (for all eyes treated) with a median follow up period of approximately 2 years. A series of IAC patients from Will's Eye Hospital in 2019 showed that 88% of Group D eyes were salvaged and 100% of Group B and C eyes. However, a meta-analysis of all IAC series published in the literature showed lower success rates for all treated eyes and advanced eyes (Groups D and E) of *. The reasons for the differences in globe salvage rates in published series and between centers is not known.
Other notable studies
- A prospective study of all retinoblastoma cases treated with selective intraophthalmic melphalan was performed at Bascom Palmer Eye Institute in Miami, FL between 2008 - 2009 [20]. A total of 26 IA infusions of melphalan were performed to treat 17 advanced tumors in 15 patients. All eyes were RE Vb at presentation (ICRB Group D). All tumors had evidence of vitreous seeding. All but one eye had not responded to aggressive multimodal chemotherapy which was otherwise scheduled for enucleation. Mean follow-up was 8.6 months (range 3-12 months). IA chemotherapy was successfully delivered in all 26 treatment sessions (25 via OA, 1 via OA originating from the MMA). Overall ocular preservation rate was 76.5% (13 of 17 eyes). The mean number of IA treatment sessions was 1.4 per eye (range 1-2). All four cases that required enucleation were treated with IA melphalan doses of 3 or 5 mg, whereas all eyes that received 7.5 mg of melphalan intra-arterially achieved tumor control (mean follow-up of subgroup treated with higher melphalan dose of 7.5mg was 6.2 months, range 3-8 months). There were no tumor recurrences during the follow-up period.
- A retrospective interventional series of 17 patients treated with intra-arterial chemotherapy in Philadelphia, PA was done at the Wills Eye Institute [9]. 17 eyes of 17 patients were treated with a total of 38 intra-arterial treatments. Intra-arterial chemotherapies used included melphalan 5mg in all cases and additional carboplatin 30mg in a select number of cases. Use of carboplatin was later discontinued due to observation of possible ophthalmic and vascular attenuation consistent with the sclerosing effect of platinum-based drugs. All catheterizations were unilateral. Intra-arterial therapy was used as a primary treatment in 13 cases and 4 eyes has previously failed other therapies. Stage at presentation for naïve eyes included RE Vb – 6 eyes, RE IVa – 5 eyes, RE IIIb – 2 eyes (ICRB Group E – 6 eyes, Group D – 5 eyes, Group C – 2 eyes). Per authors, the four eyes that had previously failed systemic chemotherapy could not be staged due to recurrence of disease. Catheterization was successful 97.3% (37 of 38 attempts). The eye that failed catheterization was due to an anomalous ICA and was treated with systemic chemoreduction. Following intra-arterial treatment, complete response was observed in 88% (14 eyes) and partial response in 12% (2 eyes). Eyes with a complete response that followed-up for a minimum of one year (n=10 eyes) showed no solid tumor recurrence. Vitreous seeds were present in 56% (9 eyes) and subretinal seeds in 69% (11 eyes). Of the 11 eyes with subretinal seeds, 82% (9 eyes) had a complete response, 9% (1 eye) had a partial response and 9% (1 eye) had a recurrence. Of the 9 eyes with vitreous seeds, 67% (6 eyes) had a complete response, 22% (2 eyes) had a partial response and 11% (1 eye) had recurrence. Of the 12 eyes managed with IA chemotherapy as a primary treatment, globe salvage was achieved in 67%; ICRB Group C or D showed 100% salvage whereas Group E had 33% salvage. Among the 4 eyes that had previous failed systemic chemotherapy, globe salvage was 50%.
- The Philadelphia group further assessed the efficacy of less than three cycles of Intra-arterial chemotherapy with a retrospective, nonrandomized, interventional case series [10]. This had previously been studied and reported by Abramson and colleagues [2][11] with longer follow-up, but nevertheless, this series (with limited follow-up time) adds to the growing body of literature on intra-arterial therapy. Once again, melphalan 5 mg was used in all cases and additional carboplatin 30mg was used in one case. This report included 8 eyes, 6 with IA used as a primary treatment and 2 eyes that had previously failed other treatment. Stage at presentation for naïve eyes was ICRB Group E – 1 eye, Group D – 3 eyes, Group C – 2 eyes. The two eyes that had previously failed other treatment could not be staged per authors due to recurrence of disease. 3 eyes were treated with a single cycle of IA chemotherapy and 5 eyes were treated with 2 cycles. After intra-arterial chemotherapy, there was a complete tumor response in 88% (7 eyes) and partial response in 13% (1 eye). Mean follow-up was 13 months. Among naïve eyes treated with intra-arterial chemotherapy, globe salvage was achieved in 100% (5 of 5 eyes) of Group C or D eyes, and 0% (0 of 1) Group E eyes. Among eyes that had previously failed other treatments, intra-arterial chemotherapy salvaged 50% (1 of 2 eyes). Among all eyes in this study, globe salvage was achieved in 75%.
- A retrospective review of 13 consecutive patients with retinoblastoma who received a total of 30 intra-arterial chemotherapy infusions of melphalan (0.35mg/kg but not exceeding 7.5mg) was completed between 2008 and 2010 in Switzerland [19]. Stage at presentation was ICRB Group D – 12 eyes and Group B – 1 eye. The success rate of ophthalmic artery catheterization was 96.8% (30 of 31); one infusion was unable to be delivered secondary to vasospasm of the ICA. Mean follow-up was 7 months. Tumor regression was observed in all eyes, with a complete response in 6 eyes. Enucleation and external beam radiation were avoided in all but one eye who required external beam radiation for a persistent papillary tumor.
RetCam images
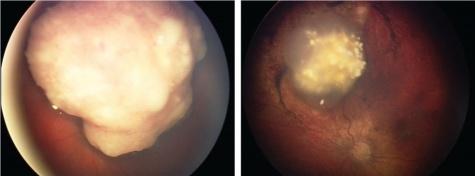
Primary treatment
Advanced retinoblastoma successfully treated with intra-arterial therapy as a primary treatment. Photo courtesy of David Abramson, MD.
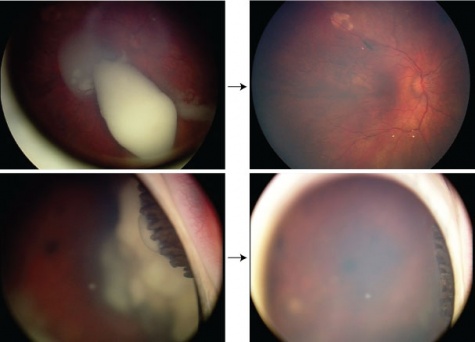
Salvage therapy
Advanced retinoblastoma involving ciliary body that failed systemic chemoreduction successfully salvaged with intra-arterial therapy. Photo courtesy of David Abramson, MD.
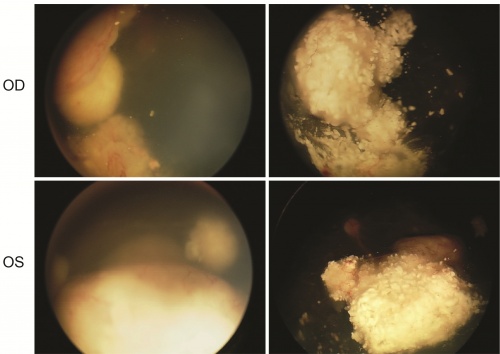
Tandem therapy via alternative route
Bilateral retinoblastoma successfully treated with tandem intra-arterial therapy only via the middle meningeal artery (MMA). Photo courtesy of David Abramson, MD.
Catheterization of the ophthalmic artery
Video courtesy of Pierre Gobin, MD.
Clinical Outcomes Compared to External Beam Radiation and Systemic Chemotherapy
External Beam Radiation
External beam radiotherapy (EBR) is the oldest form of ocular salvage therapy. The Reese-Ellsworth system used to classify intraocular retinoblastoma is based on the likelihood of preserving the eye with EBR therapy (RE I - ‘very favorable’, RE V – ‘very unfavorable’). EBR is able to save up to 53.4% of advanced (RE Vb) eyes at 10 year follow-up but the majority (52.4%) of patients are plagued with significant side effects including cataract, chronic dry eye, keratopathy, vitreous hemorrhage, radiation retinopathy, radiation optic neuropathy and facial bone hypoplasia [36]. EBR also increases the risk of second non-ocular cancers (sarcomas) particularly in patients with heritable retinoblastoma [37] [38] [39]. Due to these side effects, in the 1990s, systemic chemotherapy became the most common first line treatment for retinoblastoma. EBR is still used in select cases as a salvage therapy after failure of systemic chemoreduction and for the treatment of extra ocular retinoblastoma.
Systemic Chemotherapy
For low grade retinoblastoma, systemic chemotherapy followed by local therapy, also referred to as ‘chemoreduction’, can achieve tumor control. In RE Group I-IV eyes without seeding, chemoreduction can offer favorable outcomes, with Murphee et al. reporting 100% tumor control for these eyes [40]. Friedman et al. report 100% tumor control for RE Group I-III eyes with chemoreduction and focal therapy [41]. Beck et. al. report systemic chemoreduction was effective in 71% of RE I-III eyes [42]. In Brazil, Antonelli et al. report that for RE I-III eyes 50% of unilateral and 79.1% of bilateral cases were effectively treated with systemic chemoreduction [43]. Shields et al. report RE Group I-IV eyes treated with chemoreduction and focal therapy required additional EBR in 10% of cases and enucleation in 15% at 5 year follow-up [14].
In contrast, nearly all studies investigating the use of systemic chemoreduction in advanced intraocular retinoblastoma (RE IV and V) report less favorable outcomes with study authors commenting: there are ‘disappointing results in advanced eyes’ [42] and ‘more effective therapy is required for RE IV or V eyes’ [41]. Some groups have suggested that prolonged systemic chemotherapy should only be considered for RE I-III eyes given the poor results and excessive toxicity seen in more advanced eyes [44]. Furthermore, subretinal or vitreous seeding portends a particularly poor prognosis with Kaneko et. al noting that ‘systemic chemotherapy can rarely cure vitreous seeding’ [45] and Shields et. al. demonstrating that one of the most important predictive factors for chemoreduction failure is the presence of vitreous or subretinal seeding [14]. Friedman et al. reports that for RE Group IV eyes only 66% and 47% of RE V eyes avoided enucleation or EBR after systemic chemotherapy [41]. Cohen et al. report the 2 year event-free (EBR or enucleation) survival for Group D eyes as 34% [46]. Antonelli et al. state with RE IV-V eyes salvage was seen in 40.7% of bilateral cases and 0% of unilateral cases [43]. From China, a recent report showed a 40% globe salvage rate for Group D and 0% globe salvage rate for Group E eyes treated with systemic chemoreduction [47]. Shields et. al. report that among RE V eyes 47% required EBR and 53% required enucleation at 5 years.
Complications
Given multiple centers are now performing intra-arterial therapy, there is a growing body of literature reporting the side effects of intra-arterial treatment.
Systemic side effects
There have been no reports of deaths, stroke or other neurologic complications at any center [6][7][11][12][15][19][20][21]. To date, no child has developed a second cancer, however, it is inevitable that children with germline retinoblastoma will develop additional cancers even in the absence of radiation or systemic chemotherapy [1]. The New York group has reported two cases of metastatic disease with both patients currently in remission [1][11]. Significant bronchospasm has been reported during 8% of procedures in New York[11], noted by other groups [19][20][34], thought to be related to depth of anesthesia [1], and is now effectively treated with injection of epinephrine bitartrate [11]. Vasospasm of the ophthalmic artery and/or other vessels has also been reported during the angiographic procedure[11][19][34]. Abramson and colleagues report one episode of transient vascular occlusion of the superficial femoral artery that recanalized with aspirin therapy after 1 week [11], and a self limited groin hematoma was noted by the Miami, FL group [20].
Significant neutropenia (CTCAE v3.0, grade 3 or higher) has been reported in 11.4% of cases from New York, mostly when the melphalan dose exceeded 0.4mg/kg of body weight [11]. In New York, one patient with extensive previous systemic chemotherapy required hospitalization for neutropenic fever and transfusion of blood products on one occasion. Significant neutropenia was reported after 13.3% of treatment sessions from Miami, with one patient admitted to the hospital with fever and discharged the next day after a negative work-up [20]. Transient cytopenia was noted after 15.7% of treatments in a series from Philadelphia, with spontaneous recovery in all patients without any need for transfusion of blood products [34]. In comparison to systemic chemotherapy, no child has required the placement of a port, and additional adverse effects seen with systemic chemotherapy including nausea, vomiting, alopecia, severe myelosuppresion [48], failure to thrive [49], otoxicity [50] and acute myelogenous leukemia [51] have not been observed.
Ocular adverse effects
Transient ocular side effects, including periocular edema, blepharoptosis, transient hyperemia in the distribution of the supratrochlear artery ( present in up to 16% of patients [52], now reduced with administration of a vasoconstrictive agent to the ipsilateral nasal mucosa), epiphora, and temporary loss of nasal lashes have been observed, but never as permanent side effects [1]. Shields et al. report similar temporary ocular side effects with the addition of a case of transient orbital congestion with extraocular muscle dysfunction [34].
Most recently, attention has been drawn to possible intraocular vascular events after IA chemotherapy. In many cases, eyes have received extensive previous treatments including systemic chemotherapy, laser therapy, cryotherapy, and have had a history retinal detachment with some cases corrected surgically. These factors make it difficult to attribute changes in the retinal vasculature to tumor effect, retinal detachment, previous treatment, IA drug effect, improper delivery of IA chemotherapy (including possible ‘wedge flow’ which causes rapid alteration of flow and pressure in the cannulated vessel resulting in retinal hemorrhages), or a combination of the aforementioned factors [1][2].
In New York, avascular retinopathy was reported in 4 RE V eyes which would have otherwise been enucleated [11]. In Philadelphia, clinically visible occlusive vasculopathy of the ophthalmic artery was observed in 3 cases with fundus features of central retinal artery obstruction in one case, multifocal branch retinal artery obstruction in all cases and evidence of choroidal atrophy in 2 cases [34]. However, the group notes that in one case the ophthalmic artery obstruction and fundus findings resolved completely at 1 month. The Philadelphia group reports that focal or diffuse RPE mottling was detected in 9 eyes and among those followed for ≥ 6 months choroidal atrophy was noted in 44% (4 of 9 eyes) [34]. In Switzerland, 15% (2 of 13 eyes) showed sectoral choroidal atrophy and one eye also had retinal emboli during a ‘prolonged and particularly difficult cannulation of the ophthalmic artery’ [19]. In Miami, 15% (4 eyes) had delayed vitreous hemorrhage that was discovered 3 weeks after IA treatment, but the exact time of the hemorrhage remains unknown [20]. Additionally, in Miami, 3.8% (1 eye) showed cotton wool spotting and blanching consistent with retinal ischemia which presented with intermittent visual symptoms one day after IA treatment [20]. Further studies are needed to elucidate the causes of these vascular events given the multiple factors, including variation in angiographic procedure and extensive previous treatment before IA therapy, that could account for the changes observed in these advanced retinoblastoma eyes.
Visual Function
Abramson et al. report on visual function before and after IA treatment as measured by electroretinogram (ERG) testing [33]. The amplitude of the response to a 30-Hz flicker, measured in mV, is compared after treatment sessions, with an absolute change of ≥ 25mV considered to be a notable change. Overall, 70% of eyes have shown stable ERG readings before and after IA treatment, 20% show improvement and 10% show worsening responses [1][2][6][7][12][33][35]. In eyes that present with retinal detachment, approximately 30% show improvement in ERG testing [1][2].
Only one other center in Switzerland has reported on visual acuity outcomes [19]. Although 13 eyes were treated, only 10 eyes were available for testing due to young patient age and limited follow-up. All but one eye had vision better than 20/400, one half had vision better than 20/100 and two eyes had vision of 20/32.
Radiation exposure
One report examined the radiation exposure under fluoroscopy with intra-arterial therapy [53]. The authors found that the crystalline lens is the structure at greatest risk for radiation side effects after IA therapy. From previous reports, at a cumulative exposure of 0.5 Gy, the lens is at risk for the development of cataract. The estimated irradiation to the treatment eye lens is approximately 0.16 Gy per IA session. Therefore, the toxic threshold is not approached until after approximately 4 IA treatment sessions [16][53]. The amount of radiation to other organs during IA fluoroscopy including the brain, thyroid, bone marrow and gonads, remains so low during individual treatment sessions that it is unlikely the established toxic irradiation thresholds to these organs would be reached even after repeated IA treatments [16]. In a large Japanese cohort of 408 eyes with retinoblastoma treated with 1 to 18 sessions of selective (but not superselective) ophthalmic artery infusion, with long-term follow-up (median 79 months), the incidence of cataract and secondary cancers was no greater than what would otherwise be expected without fluoroscopic exposure [54].
Conclusion
Intra-arterial chemotherapy is an emerging therapy and represents a significant advance in the treatment of intraocular retinoblastoma, particularly for advanced cases. Its preliminary success is recognized worldwide, and it is currently being performed in over 31 countries. Further reports from other centers will add to the growing body of literature, and more results will be available for this promising treatment.
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Abramson, D.H., Chemosurgery for retinoblastoma: what we know after 5 years. Arch Ophthalmol. 129(11): p. 1492-4.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Abramson, D.H., et al. Lessons Learned After 5 Years (and 500 infusions) of Chemosurgery: Predictors of Success and Failure. in ISOO 2011 Biannual Meeting. 2011. Buenos Aires, Argentina.
- ↑ Reese, A.B., et al., Treatment of retinoblastoma by radiation and triethylenemelamine. AMA Arch Ophthalmol, 1954. 53(4): p. 505-13.
- ↑ Kiribuchi, M. and H. Hasegawa, [Retrograde infusion of anti-cancer drugs to the ophthalmic artery for intra ocular malignant tumors]. Iryo, 1968. 22(7): p. 772-6.
- ↑ Jump up to: 5.0 5.1 5.2 Yamane, T., A. Kaneko, and M. Mohri, The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol, 2004. 9(2): p. 69-73.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 Abramson, D.H., et al., A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology, 2008. 115(8): p. 1398-404, 1404 e1.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Abramson, D.H., et al., Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery). Ophthalmology. 117(8): p. 1623-9.
- ↑ Jump up to: 8.0 8.1 Aziz, H.A., et al., Supraselective injection of intraarterial melphalan as the primary treatment for late presentation unilateral multifocal stage Vb retinoblastoma. Retina. 30(4 Suppl): p. S63-5.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 Shields, C.L., et al., Intra-arterial Chemotherapy for Retinoblastoma: Report No. 1, Control of Retinal Tumors, Subretinal Seeds, and Vitreous Seeds. Arch Ophthalmol. 129(11): p. 1399-406.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 Shields, C.L., et al., Minimal Exposure (One or Two Cycles) of Intra-arterial Chemotherapy in the Management of Retinoblastoma. Ophthalmology.
- ↑ Jump up to: 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 11.21 Gobin, Y.P., et al., Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 129(6): p. 732-7.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 Abramson, D.H., et al., Bilateral superselective ophthalmic artery chemotherapy for bilateral retinoblastoma: tandem therapy. Arch Ophthalmol. 128(3): p. 370-2.
- ↑ Reese, A.B. and R.M. Ellsworth, The evaluation and current concept of retinoblastoma therapy. Trans Am Acad Ophthalmol Otolaryngol, 1963. 67: p. 164-72.
- ↑ Jump up to: 14.0 14.1 14.2 Shields, C.L., et al., Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol, 2002. 133(5): p. 657-64.
- ↑ Jump up to: 15.0 15.1 15.2 Abramson, D.H., et al., Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol.
- ↑ Jump up to: 16.0 16.1 16.2 Shields, C.L., et al. Intra-arterial Chemotherapy for Retinoblastoma: Exposures, Tumor Control, Complications - New Observations. in ISOO 2011 Biannual Meeting. 2011. Buenos Aires, Argentina.
- ↑ Shields, C.L., et al., Effect of Intraarterial Chemotherapy on Retinoblastoma-Induced Retinal Detachment. Retina.
- ↑ Inomata, M. and A. Kaneko, Chemosensitivity profiles of primary and cultured human retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res, 1987. 78(8): p. 858-68.
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 Munier, F.L., et al., Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 31(3): p. 566-73.
- ↑ Jump up to: 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 Peterson, E.C., et al., Selective ophthalmic artery infusion of chemotherapy for advanced intraocular retinoblastoma: initial experience with 17 tumors. J Neurosurg. 114(6): p. 1603-8.
- ↑ Jump up to: 21.0 21.1 Vajzovic, L.M., et al., Supraselective intra-arterial chemotherapy: evaluation of treatment-related complications in advanced retinoblastoma. Clin Ophthalmol. 5: p. 171-6.
- ↑ Laurie, N.A., et al., Topotecan combination chemotherapy in two new rodent models of retinoblastoma. Clin Cancer Res, 2005. 11(20): p. 7569-78.
- ↑ Jump up to: 23.0 23.1 Schaiquevich, P., et al., Pharmacokinetic Analysis of Topotecan after Superselective Ophthalmic Artery Infusion and Periocular Administration in a Porcine Model. Retina.
- ↑ Mallipatna, A.C., et al., Periocular topotecan for intraocular retinoblastoma. Arch Ophthalmol. 129(6): p. 738-45.
- ↑ Marr, B., et al. Intra-arterial Chemotherapy using Simultaneous Multi Agent Chemotherapy, Three Drugs, For Rescue of Eyes with Intraocular Retinoblastoma. in ISOO 2011 Biannual Meeting. 2011. Buenos Aires, Argentina.
- ↑ Jump up to: 26.0 26.1 Patel, M., et al., Intra-arterial and oral digoxin therapy for retinoblastoma. Ophthalmic Genet. 32(3): p. 147-50.
- ↑ Jump up to: 27.0 27.1 Klufas, M.A., et al. Selective Intra-arterial Chemotherapy as a Treatment for Intraocular Retinoblastoma: Alternatives to Direct Ophthalmic Artery Cannulation. in ISOO 2011 Biannual Meeting. 2011. Buenos Aires, Argentina.
- ↑ Hayreh, S.S., The Orbital Vessels of Rhesus Monkeys. Exp Eye Res, 1964. 3: p. 16-30.
- ↑ Hayreh, S.S., Orbital vascular anatomy. Eye (Lond), 2006. 20(10): p. 1130-44.
- ↑ Hayreh, S.S. and R. Dass, The Ophthalmic Artery: I. Origin and Intra-Cranial and Intra-Canalicular Course. Br J Ophthalmol, 1962. 46(2): p. 65-98.
- ↑ Cooke, D., et al., Zygomatico-orbital intra-arterial melphalan infusion for intraocular retinoblastoma. J Neurointerv Surg.
- ↑ Pham, C.T., et al., Access to the ophthalmic artery by retrograde approach through the posterior communicating artery for intra-arterial chemotherapy of retinoblastoma. Neuroradiology.
- ↑ Jump up to: 33.0 33.1 33.2 Brodie, S.E., et al., Persistence of retinal function after selective ophthalmic artery chemotherapy infusion for retinoblastoma. Doc Ophthalmol, 2009. 119(1): p. 13-22.
- ↑ Jump up to: 34.0 34.1 34.2 34.3 34.4 34.5 34.6 Shields, C.L., et al., Intra-arterial Chemotherapy for Retinoblastoma: Report No. 2, Treatment Complications. Arch Ophthalmol. 129(11): p. 1407-15.
- ↑ Jump up to: 35.0 35.1 Abramson, D.H., Super selective ophthalmic artery delivery of chemotherapy for intraocular retinoblastoma: 'chemosurgery' the first Stallard lecture. Br J Ophthalmol. 94(4): p. 396-9.
- ↑ Abramson, D.H., et al., Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol, 2004. 122(9): p. 1316-23.
- ↑ Abramson, D.H., et al., Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology, 2001. 108(10): p. 1868-76.
- ↑ Wong, F.L., et al., Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. Jama, 1997. 278(15): p. 1262-7.
- ↑ Roarty, J.D., I.W. McLean, and L.E. Zimmerman, Incidence of second neoplasms in patients with bilateral retinoblastoma. Ophthalmology, 1988. 95(11): p. 1583-7.
- ↑ Murphree, A.L., et al., Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch Ophthalmol, 1996. 114(11): p. 1348-56.
- ↑ Jump up to: 41.0 41.1 41.2 Friedman, D.L., et al., Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol, 2000. 18(1): p. 12-7.
- ↑ Jump up to: 42.0 42.1 Beck, M.N., et al., First-line chemotherapy with local treatment can prevent external-beam irradiation and enucleation in low-stage intraocular retinoblastoma. J Clin Oncol, 2000. 18(15): p. 2881-7.
- ↑ Jump up to: 43.0 43.1 Antoneli, C.B., et al., Treatment of retinoblastoma patients with chemoreduction plus local therapy: experience of the AC Camargo Hospital, Brazil. J Pediatr Hematol Oncol, 2006. 28(6): p. 342-5.
- ↑ Kim, J.H., et al., Clinical result of prolonged primary chemotherapy in retinoblastoma patients. Korean J Ophthalmol, 2003. 17(1): p. 35-43.
- ↑ Kaneko, A. and S. Suzuki, Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol, 2003. 33(12): p. 601-7.
- ↑ Cohen, V.M., J. Kingston, and J.L. Hungerford, The success of primary chemotherapy for group D heritable retinoblastoma. Br J Ophthalmol, 2009. 93(7): p. 887-90.
- ↑ Qian, J., et al., [Clinical therapeutic efficiency of chemoreduction and local therapy for children with retinoblastoma]. Zhonghua Yan Ke Za Zhi. 46(4): p. 312-6.
- ↑ Benz, M.S., et al., Complications of systemic chemotherapy as treatment of retinoblastoma. Arch Ophthalmol, 2000. 118(4): p. 577-8.
- ↑ Rizzuti, A.E., I.J. Dunkel, and D.H. Abramson, The adverse events of chemotherapy for retinoblastoma: what are they? Do we know? Arch Ophthalmol, 2008. 126(6): p. 862-5.
- ↑ Jehanne, M., et al., Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatr Blood Cancer, 2009. 52(5): p. 637-43.
- ↑ Levin, M.H., D.S. Gombos, and J.M. O'Brien, Intra-arterial Chemotherapy for Advanced Retinoblastoma: Is the Time Right for a Prospective Clinical Trial? Arch Ophthalmol. 129(11): p. 1487-9.
- ↑ Marr, B., et al., Spontaneously Resolving Periocular Erythema and Ciliary Madarosis Following Intra-arterial Chemotherapy for Retinoblastoma. Middle East Afr J Ophthalmol. 17(3): p. 207-9.
- ↑ Jump up to: 53.0 53.1 Vijayakrishnan, R., et al., Irradiation toxic effects during intra-arterial chemotherapy for retinoblastoma: should we be concerned? Arch Ophthalmol. 128(11): p. 1427-31.
- ↑ Suzuki, S., et al., Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology. 118(10): p. 2081-7.