Internal Limiting Membrane Dystrophy (ILMD)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Internal limiting membrane dystrophy (also known as Familial Müller cell sheen dystrophy or MCSD) is a rare genetic retinal dystrophy characterized by a classic macular sheen associated with schisis and cystic cavities seen in the internal limiting membrane (ILM) of the posterior pole. [1]
Disease Entity
History
The first case of ILMD was described in fifteen members of a single family by Dalma-Weiszhausz, et. al. in 1991. He examined 45 members of a 4-generation family and noted a retinal dystrophy characterized by a glistening inner retinal surface throughout the posterior pole, with vision loss occurring later in life due to superficial polycystic retinal edema and folds.[2] Since then, several case series and case reports describing the same disease entity have been published in the literature. [1][3][4][5][6][7][8]
Pathophysiology and Genetics
A primary defect in Müller cells is thought to be the main cause behind the pathological and clinical findings of ILMD. This defect is suspected to cause abnormal synthesis of the basal lamina fibrils of the internal limiting membrane (ILM) which, in turn, leads to detachment and formation of schisis cavities.[1][2] The Müller cell hypothesis is supported by the similarity between ILMD and X-linked juvenile retinoschisis (XLRS). XLRS is a well-studied disease entity that is also believed to be caused by a primary defect in Müller cells.[9] Both ILMD and XLRS are characterized by diminished b-wave amplitude on ERG and similar retinal polycystic cavities, although they are less widespread and much more obvious in XLRS. The b-wave on ERG is thought to be mainly generated by Müller cells, further suggesting that Müller cells are primarily defected in this condition.[4] In addition, histopathologically identical retinal filaments of similar sizes, thought to be basal lamina components produced by defective Müller cells, can be found adjacent to areas of retinal schisis in both ILMD and XLRS.[1]
Other hypotheses, such as mutations in genes coding for structural basal lamina proteins or a primary vitreal defect leading to vitreoretinal traction, have also been suggested as possible causes for ILMD. However, there has been insufficient clinical or histopathological evidence to support these theories. The cause of the classic glistening appearance of the posterior pole seen clinically is still not fully understood, but it is believed to be caused by a change in the refractive index of the ILM causing it to become less transparent and more refractive to light.[1] To date, there have been two reliable published pedigrees. The first pedigree, by Dalma-Weiszhausz, et al., analyzed 45 members of a single family that were affected and had classical imaging and clinical findings pertaining to ILMD.[2] The second pedigree, by Polk. et al., analyzed 15 members of a single family where 5 were found to have classic features of ILMD.[1] Both pedigree analyses suggested an autosomal dominant form of inheritance, although mitochondrial inheritance with incomplete penetrance could not be excluded. [1][2] In addition, sporadic forms of ILMD have also been reported numerously in the literature. [3][6][7][8]
Diagnosis
The diagnosis of ILMD is typically made using a combination of both clinical exam findings and imaging results.
Clinical presentation
The majority of patients detected with ILMD are asymptomatic. Visual acuity loss in reported cases was mostly seen starting in the 5th -8th decade of life. Most occurred due to a concomitant inciting event such as intraocular surgery, idiopathic central serous retinopathy, or vitreous traction secondary to age-related vitreous changes.[1] However, younger cases with affected visual acuity have been reported in the literature with the youngest being an 18-year-old male.[3] The disease course can manifest with bilateral or unilateral progression in severity.[6]
Fundus ophthalmoscopy
ILMD on fundoscopic exam is characterized by a classic bilateral and symmetric glistening inner retinal sheen that is usually more pronounced at the posterior pole. The sheen is widespread in nature, superficial to the level of the retinal vessels, and associated with retinal folds throughout the fundus.[1][2][6]
Imaging
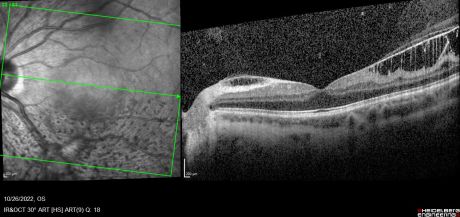
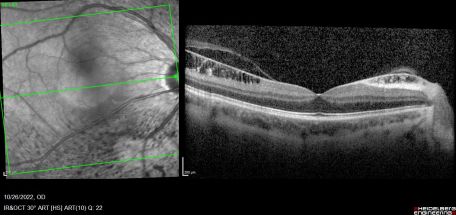
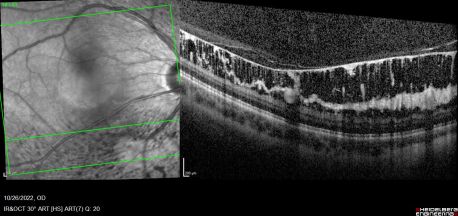
Combined cone and rod electroretinography (ERG) shows selective diminution of the b-wave (also known as a negative-ERG). Fluorescein angiography tends to show no abnormalities in the early phase of the angiogram but may show hyper-fluorescence and vascular leakage in late phases. [1] [3] Adaptive optics at 2- and 4-degrees eccentricity show normal cone cell count, photoreceptor packing geometry and intercellular spacing.[3] Optical coherence tomography of affected areas shows schisis retinal cavities mainly in the inner retinal layers but can extend to outer layers in later stages of the disease.[6]
Histopathology
While histopathological evaluation is not routinely done for patients with suspected ILMD, specimens from enucleated eyes have shown characteristic findings. In normal histology, the ILM consists of a periodic acid-Schiff-positive membrane that is composed of two identified layers.[10] The outermost layer consists of a basal lamina that acts as a basement membrane for Müller cells and is also synthesized by them, while the innermost layer has been describes as a vitreal layer that is composed of vitreal fibrils and mucopolysaccharides.[11] However, in ILMD, the outer layer in the ILM shows an abnormal lamina that has less staining on electron microscopy and appears detached from the overlying Müller cells. The detachment forms schisis cavities containing microfilaments and cellular debris, which are responsible for the polycystic appearance of the ILM.[1] Because of the distinct involvement of the inner layer, Foos et al. suggested the use of the term “inner limiting lamina” as a more accurate and specific term to describe the disease.[12]
Normally, the ILM thickness ranges from 0.5-2.0 mm and is thickest at the fovea. In ILMD, the thickness tends to be significantly increased due to excessive production of abnormal basal lamina by defective Müller cells. This is more pronounced at the posterior pole as it naturally contains thicker ILM. [4] [8]As the disease progresses, cystic spaces are seen to involve outer layers and may eventually cause serous detachment. Foveal involvement and the appearance of macular edema is seen only when cystic spaces reach the outer nuclear layer.[5]
In addition, retinal blood vessels in patients with ILMD have been reported to show thickened multi-laminated basement membrane, endothelial cell swelling, pericyte degeneration, and lipid deposition that leads to vascular leakage and cystoid edema. These vascular changes appear similar to those found in diabetic retinopathy. Nonetheless, these changes appear to be secondary, as they are not evident in all patients with ILMD and the abnormal glistening reflex tends to precede the appearance of vascular leakage.[1] Furthermore, Carlson et al. demonstrated that the basement membrane of Müller cells are continuous with that of the entire retinal capillary complex.[13] Müller cell processes also have a major role in forming the inner-blood-retinal barrier, thus further explaining how a possible defect in Müller cells may cause secondary damage to the basement membrane of the retinal vascular capillaries.[14]
Differential diagnosis
The differential for this disease is limited but include the following:
Vitreoretinal traction and idiopathic epiretinal membranes can both have a similar posterior sheen to ILMD, however, they are both typically unilateral.[1] In addition, in ILMD, there is diffuse ILM schisis seen on imaging, meanwhile vitreoretinal traction tends to have more focal schisis. Epiretinal membranes also tend to cause intra retinal fluid as opposed to retinal schisis. [15][16]
Fenestrated Sheen Macular Dystrophy (FSMD) is characterized by a yellow sheen with red fenestrations that is limited to the macula. This sheen is usually deep to the retinal vessels and patients develop a progressive annular hypopigmentation around the area of sheen at the level of the retinal pigmented epithelium. Eventually a hyperpigmented area centrally appears giving rise to a “bull’s eye” appearing lesion. Unlike FSMD, the sheen in ILMD is more widespread and is superficial to the retinal vessels. It also has no red fenestrations and there is usually no evidence of an annular hypopigmented area. [1][17]
Other important differentials to also consider include x-linked retinoschisis, degenerative retinoschisis, and Alport syndrome.
Management
There is no known definitive treatment for ILMD. However, most patients are asymptomatic and do not require treatment until later in life when their vision starts to be affected, usually in the 5th-8th decades. Regular follow up, in addition to patient education and counseling regarding disease course and progression, is of vital importance for optimal delivery of medical care. The use of non-steroidal anti-inflammatory agents, acetazolamide, steroids, and laser photocoagulation have demonstrated no significant visual improvement. Vitrectomy has also been shown to not yield significant improvement,[1] [8] though Renner et al. described a successful vitrectomy with internal limiting membrane peel that resulted in significantly improved best corrected visual acuity in one patient with ILMD. This case also demonstrated that oral acetazolamide could produce temporary improvement in visual acuity but this outcome may differ significantly between individuals.[3]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Polk TD, Gass JDM, Green WR, Novak MA, Johnson MW. Familial Internal Limiting Membrane Dystrophy: A New Sheen Retinal Dystrophy. Archives of Ophthalmology. 1997;115(7):878-885. doi:10.1001/ARCHOPHT.1997.01100160048007
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Dalma-Weiszhausz J, Chacón-Camacho O, Chevez-Barrios P, et al. AUTOSOMAL DOMINANT MÜLLER CELL SHEEN DYSTROPHY: Clinical, Histopathologic, and Genetic Assessment in an Extended Family With Long Follow-Up. Retina. 2022;42(5):981-991. doi:10.1097/IAE.0000000000003413
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 Oli A, Balakrishnan D. Multimodal imaging and adaptive optics in internal limiting membrane dystrophy. BMJ Case Reports CP. 2020;13(8):e234960. doi:10.1136/BCR-2020-234960
- ↑ Jump up to: 4.0 4.1 4.2 Kellner U, Kraus H, Heimann H, Helbig H, Bornfeld N, Foerster MH. Electrophysiological evaluation of visual loss in Müller cell sheen dystrophy. Br J Ophthalmol. 1998;82(6):650. doi:10.1136/BJO.82.6.650
- ↑ Jump up to: 5.0 5.1 Franco-Cardenas V, Dalma-Weiszhausz J, Martinez-Munoz R, Dalma A. SD-OCT Progressive Alterations in a Family Affected with Müller Cell Sheen Dystrophy. Invest Ophthalmol Vis Sci. 2013;54(15):5921-5921.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Renner AB, Radeck V, Kellner U, Jägle H, Helbig H. Ten-year follow-up of two unrelated patients with Müller cell sheen dystrophy and first report of successful vitrectomy. Doc Ophthalmol. 2014;129(3):191-202. doi:10.1007/S10633-014-9463-9
- ↑ Jump up to: 7.0 7.1 Kishk H. Internal limiting membrane dystrophy: a case series study of a rarely reported retinal dystrophy. Journal of the Egyptian Ophthalmological Society. 2017;110(4):118. doi:10.4103/EJOS.EJOS_43_17
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Parida H, Kannan N, Rathinam S. Imaging of Muller cell sheen dystrophy. Indian J Ophthalmol. 2020;68(3):533-535. doi:10.4103/IJO.IJO_930_19
- ↑ Yanoff M, Rahn EK, Zimmerman LE. Histopathology of Juvenile Retinoschisis. Archives of Ophthalmology. 1968;79(1):49-53. doi:10.1001/ARCHOPHT.1968.03850040051014
- ↑ Spencer WH. Ophthalmic pathology: an atlas and textbook, Volume 3, Issue 1986. Published online 1985:2860. Accessed October 29, 2022. https://books.google.com/books?id=PSBPAQAAIAAJ&pgis=1
- ↑ HOGAN MJ. The Vitreous, Its Structure, and Relation to the Ciliary Body And Retina : Proctor Award Lecture. Invest Ophthalmol Vis Sci. 1963;2(5):418-445.
- ↑ Vitreoretinal juncture; topographical variations - PubMed. Accessed October 29, 2022. https://pubmed.ncbi.nlm.nih.gov/4561129/
- ↑ Carlson EC. Human retinal capillary basement membrane leaflets are morphologically distinct: A correlated TEM and SEM analysis. Exp Eye Res. 1989;49(6):967-981. doi:10.1016/S0014-4835(89)80020-X
- ↑ Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61(5):651-678. doi:10.1002/GLIA.22477
- ↑ Stevenson W, Prospero Ponce CM, Agarwal DR, Gelman R, Christoforidis JB. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin Ophthalmol. 2016;10:527. doi:10.2147/OPTH.S97722
- ↑ Stalmans P, Duker JS, Kaiser PK, et al. Oct-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina. 2013;33(10):2003-2011. doi:10.1097/IAE.0B013E3182993EF8
- ↑ O’donnell FE, Welch RB. Fenestrated Sheen Macular Dystrophy: A New Autosomal Dominant Maculopathy. Archives of Ophthalmology. 1979;97(7):1292-1296. doi:10.1001/ARCHOPHT.1979.01020020034007