Implantable Collamer Lens
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Posterior Chamber Phakic Intraocular lenses
Implantable collamer lenses are a type of posterior chamber phakic intraocular lens (pIOL). pIOL are usually used for high degrees of refractive error or when corneal refractive surgery is contraindicated. Advantages of pIOLs over corneal refractive surgery also include quicker recovery and reversibility. Posterior chamber pIOLs may provide less risk in comparison to anterior chamber (AC) IOLs because they are less visible externally and create less risk of endothelial cell loss (ECL) as they are placed further away from the cornea. [1] They are a safer alternative to refractive lens exchange which carries a risk of retinal detachment and loss of accommodation. [2] There have been a number of posterior chamber phakic intraocular lenses on the market prior to the development of the implantable collamer lens. These include the Chiron Adatomed pIOL which was a rectangular, silicone plate IOL that was eventually discontinued because of high rates of AC inflammation and cataracts. [2] The PRL Phakic Refractive Lens (CIBA Vision) was another 1-piece pIOL made of hydrophobic silicone elastomer designed so the haptics would rest on the zonules and the lens would float above the natural crystalline lens; however this lens was taken off of the market due to high incidence of subluxation of the lens into the vitreous cavity secondary to zonular dehiscence. [2] Currently there is only one FDA approved posterior chamber pIOL in the US which is the EVO implantable collamer lens (ICL) (STAAR Surgical, Monrovia, CA, USA).
Implantable Collamer Lens
In 1993, STAAR Surgical (Monrovia, CA, USA) released their posterior chamber pIOL. The company created a biocompatible material called collamer which is a blend of polymer and collagen with the purpose of making the lens lighter, hydrophilic, and allowing for better exchange of gas and nutrients. [3] This patented material is made of 60% poly-hydroxymethylmethacrylate (HEMA), water (36%), and benzophenone (3.8%) and 0.2% porcine collagen. [3]
The lens underwent a number of design modifications and was FDA approved in 2005. The EVO/EVO+ ICL and its toric version were both FDA approved in the United States in March 2022 for correction of myopia and myopia with astigmatism.[4] The ICL is a rectangular one-piece plate-haptic design lens that is plano-concave. The EVO model, features an update to the design with inclusion of a central port or hole of 0.36 mm (KS-Aquaport) meant to eliminate the need for Nd:YAG iridotomy or iridectomy that was required in patients who had the previous model Visian V4 implanted. [5] [6] The port is meant to allow physiologic aqueous humor circulation. The lens also has a ultra-violet absorbing chromophore.The EVO/EVO+ ICL Model V5 is the latest ICL released and has a larger optic diameter up to 6.10. [7] [8] There is also safety data showing that the central port allowing for physiologic flow through the optic reduces the rate of anterior subcapsular cataract formation and pupillary block compared to earlier models.[9] On July 2, 2020 EVO Viva ™, STAAR’s innovative presbyopia correcting implantable Collamer® lens (“ICL”), was approved for sale. STAAR received CE Mark approval of the presbyopic indication for its EVO+ Visian® ICL with Aspheric (EDOF) Optic, commercially marketed as “EVO Viva ”, from its European Notified Body, DEKRA . The lens is designed to work in harmony with a patient's eye for the correction or reduction of myopia and presbyopia in phakic and pseudo-phakic (post-cataract IOL) eyes to correct vision. The innovative EVO Viva lens adds near and intermediate vision correction for patients with presbyopia.
Patient Selection
Indications [10] [2] [11]
As approved by the US FDA, the Visian ICL is indicated for adults 21-45 years of age
- To correct myopia ranging from -3.0 diopters to -15.0 diopters with less than or equal to 2.5 diopters of astigmatism at the spectacle plane
- To reduce myopia ranging from greater than -15.0 diopters to -20.0 diopters with less than or equal to 2.5 diopters of astigmatism at the spectacle plane
- Anterior chamber depth (ACD) 3.00 mm or greater is required for implantation.
- Stable refractive history within 0.5 diopter for 1 year is required prior to implantation.
The Visian Toric ICL is indicated for use in patients 21-45 years of age
- For the correction of myopic astigmatism with spherical equivalent ranging from -3.0D to ≤-15.0D (in the spectacle plane) with cylinder (spectacle plane) of 1.0D to 4.0D.
- For the reduction of myopic astigmatism with spherical equivalent ranging from greater than -15.0D to -20.0D (in the spectacle plane) with cylinder (spectacle plane)1.0D to 4.0D.
- With an anterior chamber depth of 3.00 mm or greater, when measured from the corneal endothelium to the anterior surface of the crystalline lens
- A stable refractive history (within 0.5D for both spherical equivalent and cylinder for 1 year prior to implantation)
- The Visian TICL is intended for placement in the posterior chamber (ciliary sulcus) of the phakic eye.
Although the FDA approval is pending for The Evo Viva ICL at present, the Directions for Use (DFU) lists the indications as: EVO Viva TM ICL (Implantable Collamer ® Lens) with Aspheric (EDOF) Optic is indicated for use in phakic eye treatment in patients 21– 60 years of age and pseudophakic eye treatment in patients with monofocal IOLs with and without cylinder correction 21 years of age and older for:
- The correction/reduction of myopia in patients ranging from -0.5 D to -20.0 D at the spectacle plane.
- The correction/reduction of myopia with presbyopia in patients ranging from -0.5 D to -20.0 D at the spectacle plane.
- For extended depth of focus and improved near visual acuity.
- With an anterior chamber depth (ACD) equal to or greater than 2.8 mm as measured from the corneal endothelium to the anterior lens capsule.
Contraindications [10]
- Anterior chamber depth from endothelium < 3.00 mm
- Anterior chamber angle less than grade II determined by gonioscopic examination
- Pregnancy or nursing
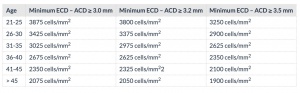
- Not meeting appropriate endothelial cell density as determined by an age-dependent minimum (range 1900- 3875 cells/mm2), see Table 1
Preoperative evaluation
Patient work-up
Prior to surgery, all patients require a complete ophthalmologic examination including uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), vertex distance, manifest and cycloplegic refraction, white to white measurement, slit-lamp biomicroscopy, intraocular pressure measurement via Goldmann applanation tonometry, corneal evaluation including central corneal thickness measurement, endothelial cell count, as well as anterior chamber depth. Furthermore patients should undergo dilated retinal examination and evaluation for the presence of retinal tears or lesions.
Lens sizing
Nomograms are available from STAAR Surgical to select the correct pIOL size based on white to white measurement and anterior chamber depth measured from the corneal endothelium to the crystalline lens (ACD-pachymetry). [10]The method used for ICL sizing in the FDA study is external white-to-white horizontal distance (with calipers) and anterior chamber depth by Scheimpflug photography. [5] [10]Another modality available to measure white to white is the Visante anterior segment OCT. Recently ultrasound biomicroscopy has been used to measure sulcus-to-sulcus distance instead of white to white. It can also measure the rise of the anterior capsule which can influence the size of the lens. [8] Accurate sizing of the lens is important as it affects the vault of the ICL. The optics of the ICL are placed in a manner to preserve a space between the crystalline lens and the ICL. It is “vaulted” over the crystalline lens. This space allows for aqueous flow over the crystalline lens, which is thought to prevent cataract formation. Ideally the size of the vault should be approximately 250-750 microns (0.5- 1.5 x corneal thickness). A larger lens is associated with higher vault. If a vault is less than 250 microns, there is risk anterior subcapsular cataract formation. A vault greater than 750 microns risks pupillary block glaucoma and crowding of the angle with possible iris chafing and resultant loss of pigment. [8]
IOL power calculation
ICL power calculation is performed through the online calculator provided by the modified vertex formula provided by STAAR surgical. The power calculation is based on several variables in the formula including preoperative manifest spherical and cycloplegic refractive error, keratometric power, corneal thickness, horizontal visible iris dimension (HVID) and central ACD. [12] ACD is measured from the corneal epithelium to the crystalline lens, as opposed to lens size calculation where the ACD is measured from the back of the cornea thus subtracting corneal thickness. Careful patient testing and measurement are essential and recommended.
Surgical Technique [10][5][13]
For the previous Visian ICL models , two YAG iridotomies placed superiorly 90 degrees apart, were performed 2 to 3 weeks prior to surgery with confirmation of the patency of the iridotomies prior to lens implantation. Alternatively, iridotomies can be performed at the time of surgery. With the EVO/EVO+ ICL and toric version peripheral iridotomies are no longer necessary.
Visian and EVO Visian ICL implantation can be performed under topical anesthesia supplemented with intracameral lidocaine or peribulbar anesthesia. The pupil is dilated preoperatively. Tropicamide 1% and phenylephrine 2.5% are preferred dilating agents; the use of longer acting agents may make it difficult to constrict the pupil at the end of the procedure. A clear temporal corneal incision measuring 3.0 to 3.2mm is made and two paracenteses incisions are made superiorly and inferiorly. Cohesive viscoelastic (Occucoat, Bausch and Lomb) is used to fill the anterior chamber before insertion of the ICL and after the lens unfolds inside the eye. Ocucoat is recommended since other viscoelastic agents may cause cataract formation when trapped between the ICL and the crystalline lens. It is important not to overfill the anterior chamber as it would make it harder to tuck the haptics. The ICL is injected anterior and parallel to the iris plane into the anterior chamber, and allowed to naturally unfold. More viscoelatic is injected anterior to the lens. The haptic footplates are then positioned under the iris. A Batlle manipulator aids in tucking the haptics of the ICL under the iris. Generally, the distal haptics (away from the incision) are positioned prior to the proximal haptics. Avoidance of contact with the central 6.0 mm of the ICL is recommended to avoid lens damage. The surgeon should avoid touching the crystalline lens and cornea (no fly zone) during the procedure to minimize risk of cataract formation and intraoperative complications. Throughout the procedure positioning instruments are used through the paracentesis ports and should be kept peripheral with minimal central touch. Viscoelastic should be removed completely after conclusion of the case in order to reduce risk of intraocular pressure (IOP) spike postoperatively. A miotic agent such as Miostat or Miochol is instilled prior to ensuring a water tight incision. The lens vault and IOP should be checked within a few hours after the procedure and the next day. The lens vault should be estimated by slit lamp biomicroscopy and should be around (1.0 +- 0.5) * corneal thickness. Patency of the iridotomy sites must be confirmed with the Visian ICL (a step not necessary when using the EVO ICL). Postoperatively, patients should be prescribed antibiotic eye drops for 1 week and topical steroid eye drops with tapering for approximately 2 to 4 weeks. In the United States, it is common and accepted practice to perform same day bilateral sequential surgery. Otherwise, the procedure can be repeated on the second eye within 1 or 2 weeks.
Visian ICL Implantation Video:
Visian Toric ICL Implantation Video:
EVO Visian ICL Implantation Video:
Outcomes
Effectiveness
In the US FDA 3-year follow up study evaluating the ICL, for patients with BSCVA of 20/20 or better at baseline, 98.4% of patients ≤ 7-D and 86.9% of patients ⬎7- to 10-D, and reported 20/20 or better uncorrected vision. For highly myopic patients (> -10D), 83.8% reported UCVA of 20/40 or better 3 years post-op. [14] Furthermore, studies comparing laser in situ keratomileusis (LASIK) to ICL implantation have found ICL to be superior in treating patients with higher degrees of myopia.[15]
Quality of Vision and Quality of Life Outcomes
ICLs seem to induce significantly fewer higher-order aberrations (HOAs) than wave front-guided LASIK and better contrast sensitivity. [15][16] In studies comparing ICL versus LASIK, patients with ICLs reported better vision–related quality of life including less activity limitations and fewer symptoms such as eye dryness or discomfort compared with patients who had undergone LASIK. [17]
Complications
The FDA clinical trial of the Visian ICL found that patients with a higher degree of pre-operative myopia had a higher rate of complications. Patients that lost 2 lines of best-spectacle-corrected visual acuity (BSCVA) occurred in the > -10 D group. [14] Furthermore all of the patients requiring cataract extraction, as well as those requiring secondary surgery to reposition the ICL occurred in the > -10D myopia group.[14] In the study, 5 eyes (1%), lost greater than 2 lines of BSCVA, with 3 of occurring secondary to nuclear opacities that developed between 1 and 3 years postop.[14] The rate of clinically significant cataract formation amongst all the groups was 0.4% for anterior subcapsular opacities and 1% of nuclear opacities.[14] With the new EVO/EVO+ ICL the rate of visually significant cataract has been cited at zero and the incidence of non visually significant Anterior Subcapsular Opacities (ASC) has remained very low.[18]
Rarer complications necessitating secondary surgical intervention that have been reported include rotation of a toric lens, pupillary block, retinal detachment or elevated IOP. [14] [19] Additional risk of complications can be attributed to the location of ICL between the iris and crystalline lens which can lead to contact with surface of the ICL and theoretically lead to pigment dispersion and/ or inflammation and possible secondary glaucoma.
References
- ↑ Kohnen T, Kook D, Morral M, Güell JL. Phakic intraocular lenses: Part 2: Results and complications. J Cataract Refract Surg. 2010;36(12):2168-2194. doi:10.1016/j.jcrs.2010.10.007
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Huang D, Schallhorn SC, Sugar A, et al. Phakic Intraocular Lens Implantation for the Correction of Myopia. A Report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(11):2244-2258. doi:10.1016/j.ophtha.2009.08.018
- ↑ Jump up to: 3.0 3.1 Lovisolo CF, Reinstein DZ. Phakic intraocular lenses. Surv Ophthalmol. 2005;50(6):549-587. doi:10.1016/j.survophthal.2005.08.011
- ↑ https://www.ophthalmologytimes.com/view/blog-a-new-era-for-refractive-surgery-in-the-united-states-staar-evo-icl-wins-fda-approval
- ↑ Jump up to: 5.0 5.1 5.2 Alfonso JF, Fernández-Vega-Cueto L, Alfonso-Bartolozzi B, Montés-Micó R, Fernández-Vega L. Five-year follow-up of correction of myopia: Posterior chamber phakic intraocular lens with a central port design. J Refract Surg. 2019;35(3):169-176. doi:10.3928/1081597X-20190118-01.
- ↑ Bhandari V, Karandikar S, Reddy JK, Relekar K. Implantable collamer lens V4b and V4c for correction of high myopia. J Curr Ophthalmol. 2015;27(3-4):76-81. doi:10.1016/j.joco.2016.01.001
- ↑ Domínguez-Vicent A, Ferrer-Blasco T, Pérez-Vives C, Esteve-Taboada JJ, Montés-Micó R. Optical quality comparison between 2 collagen copolymer posterior chamber phakic intraocular lens designs. J Cataract Refract Surg. 2015;41(6):1268-1278. doi:10.1016/j.jcrs.2014.09.050
- ↑ Jump up to: 8.0 8.1 8.2 Packer M. The implantable collamer lens with a central port: Review of the literature. Clin Ophthalmol. 2018;12:2427-2438. doi:10.2147/OPTH.S188785
- ↑ Packer M. The Implantable Collamer Lens with a central port: review of the literature.Clin Ophthalmol. 2018;12:2427-2436.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 STAAR. Visian ICL. Product Information. 2005:1-21.
- ↑ https://edfu.staar.com/edfu/5c784538fd5dd20001d67c89/ICL%20eDFU's/eDFU-0036_Rev_01_EVO%20Viva.pdf
- ↑ Chen X, Wang XY, Zhang X, Chen Z, Zhou XT. Implantable collamer lens for residual refractive error after corneal refractive surgery. Int J Ophthalmol. 2016;9(10):1421-1426. doi:10.18240/ijo.2016.10.09
- ↑ Hassaballa MA, Macky TA. Phakic intraocular lenses outcomes and complications: Artisan vs Visian ICL. Eye. 2011;25(10):1365-1370. doi:10.1038/eye.2011.187
- ↑ Jump up to: 14.0 14.1 14.2 14.3 14.4 14.5 United States Food and Drug Administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: Three-year follow-up. Ophthalmology. 2004;111(9):1683-1692. doi:10.1016/j.ophtha.2004.03.026
- ↑ Jump up to: 15.0 15.1 Igarashi A, Kamiya K, Shimizu K, Komatsu M. Visual Performance after Implantable Collamer Lens Implantation and Wavefront-Guided Laser In Situ Keratomileusis for High Myopia. Am J Ophthalmol. 2009;148(1):164-170.e1. doi:10.1016/j.ajo.2009.02.001
- ↑ Kamiya K, Igarashi A, Shimizu K, Matsumura K, Komatsu M. Visual performance after posterior chamber phakic intraocular lens implantation and wavefront-guided laser in situ keratomileusis for low to moderate myopia. Am J Ophthalmol. 2012;153(6):1178-1186.e1. doi:10.1016/j.ajo.2011.12.005
- ↑ Kobashi H, Kamiya K, Igarashi A, Matsumura K, Komatsu M, Shimizu K. Long-term quality of life after posterior chamber phakic intraocular lens implantation and after wavefront-guided laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2014;40(12):2019-2024. doi:10.1016/j.jcrs.2014.03.028
- ↑ Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059–1077
- ↑ Guri A, Lfh T, Dosage G, Contamination E, Paragraphs N. Visian® TORIC ICL (Implantable Collamer® Lens) for Myopia. 2017:2-3.