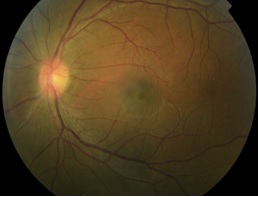
Human T-cell Lymphotropic Virus Type 1 Uveitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Human T-cell Lymphotropic virus type 1 (HTLV-1) associated uveitis, characterized by a granulomatous or nongranulomatous reaction accompanied by vitreous opacities and retinal vasculitis, can be the only manifestation of this viral infection. Although HTLV-1 is endemic to certain parts of the world (such as Japan, Melanesia, Caribbean Islands, Central and South America, and central African areas), HTLV-1 uveitis is significantly increasing in non-endemic areas due to migration, becoming a relevant agent in differential diagnosis of patients with idiopathic uveitis.
Disease Entity
Disease
Human T-cell Lymphotropic virus type 1 (HTLV-1) is a retrovirus known for its association with Adult T-cell Leukaemia/Lymphoma (ATL) and with HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis (HAM-TSP)[1] , a chronic meningomyelitis of the grey and white matter in the spinal cord, with perivascular demyelination and axonal degeneration [2].
HTLV-1 can affect the eye in multiple ways, including malignant infiltrates, corneal damage, retinal degeneration or exudation, neurophthalmic disorders, keratoconjunctivitis sicca, and uveitis (HU)[3] . HU is characterized by an anterior uveitis [4][5] with a granulomatous or nongranulomatous reaction accompanied by vitreous opacities and retinal vasculitis with rare exudative retinochoroidal alterations in one or both eyes[6]. It can be the only manifestation of the disease or be associated with HAM-TSP, in which case it is more frequent among patients with earlier onset of HAM-TSP than among patients with severe motor disability[5].
Etiology
HTLV-1 is an oncovirus of the retrovirus family, first discovered by Poiesz et al in 1980[7]. It has six reported subtypes and the great majority of infections is caused by the cosmopolitan subtype A, however, there are no reports of subtype influence on the pathogenic potential of the virus[8].
Approximately 20 million individuals are estimated to be infected with HTLV-1 worldwide, with the great majority remaining asymptomatic carriers during their lifetime[9]. It is endemic to the Japan, Melanesia, Caribbean Islands, Central and South America, and central African areas [10]. In Europe and North America the prevalence is low and limited to groups that have migrated from areas of endemicity[11] .
HU appears to be present in 112.2:100,000 HTLV-1 carriers of both sexes[12] and the prevalence rates of HU are slightly higher than those of HAM/TSP[13].
Risk Factors
The most important routes of HTLV-1 transmission is vertical transmission from mother to child, predominantly through breastfeeding (20% efficiency in transmission, correlated with individual variables such as HTLV-1 proviral load, the concordance of HLA class I type between mother and child, and the duration of breastfeeding [14]). Transmission is also possible during the intrauterine period or peripartum [15].
It is also a disease transmitted through sexual intercourse, and blood contact (the most efficient mode of transmission) [10].
There is an increasing prevalence with age and in women, especially after 40 years of age[16].
Pathophysiology
With modern molecular technology, the pathogenesis of HU has been partially unveiled[17]. The cells floating in the anterior chamber were found to be lymphocytes, the majority being CD3+ T cells, along with a few macrophages[18]. Polymerase chain reaction analysis revealed that HTLV-1 proviral DNA was detected in almost all patients with HU but not in patients seropositive for HTLV-1 and uveitis of another cause[18], which suggested a role for HLTV-1 infected cells in the pathogenesis of uveitis. Viral mRNA and HTLV-1 protein were also detected in the aqueous humor of HU patients.
HTLV-1-infected CD4+ T cell clones derived from infiltrating cells in eyes with HU were found to produce significant amounts of inflammatory cytokines, such as IL-1α, IL-2, IL-3 IL-6, IL-8, IL-10 TNF-α, GM-CSF and IFN-γ, capable of inducing immune reactions and inflammation intraocularly[19].
Also, virological analysis supported the pathogenicity of the virus in the eye, demonstrating that the HTLV-1 provirus load in patients with HU is significantly higher than that in asymptomatic carriers without uveitis [20] and that the proviral load in peripheral blood mononuclear cells correlates with the intensity of intraocular inflammation[21].
Primary prevention
Vertical transmission (especially through breastfeeding) is a very important route of dissemination; therefore, its prevention would have the most significant impact on the spread of the disease[1], [22]. In endemic areas, prenatal screening for HTLV-1 combined with counselling of seropositive mothers could help prevent and diminish the occurrence of HTLV-1 infection[1]. In the case of pregnancy, a caesarean section should be recommended to minimize the risk of perinatal transmission.
In most African countries and other areas that lack appropriate policies and infrastructures for transfusion services, blood transfusions still represent a risk of HTLV-1 infection. As such, screening for blood donor candidates would be an effective strategy in preventing HTLV-1 transmission. For countries where the disease is not endemic, selective donor recruitment is an option to consider[10].
Diagnosis
History
The patient may present with visual blurring, discomfort and floaters of acute or subacute installation. HU may be found in association with HAM/TSP or as an isolated manifestation of the viral infection[23]. There are no cases in literature of ATL developing in patients with HU[24]. An association with Graves’ disease has been reported, and in all cases HU occurred after the onset of Graves’ disease[25].
Physical examination
It is characterized by an anterior uveitis with a granulomatous or nongranulomatous reaction accompanied by vitreous opacities and retinal vasculitis[4] with rare exudative retinochoroidal alterations in one or both eyes[6]. Retinal degeneration, retinal haemorrhages, epiretinal membranes and optic nerve atrophy can occur. Anterior segment alterations might include corneal haze, scarring and neovascularization[26].
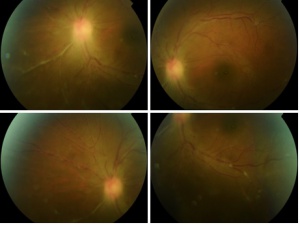
Signs
Anterior segment signs:
- Anterior granulomatous or nongranulomatous uveitis
- Haze
- Scarring
- Neovascularization[26]
- Keratoconjunctivitis sicca[3]
Posterior segment signs:
- Vitreous opacities
- Retinal vasculitis
- Exudative retinochoroidal alterations in one or both eyes[6]
- Retinal degeneration
- Retinal haemorrhages
- Epiretinal membranes
- Optic nerve atrophy (rare).
Symptoms
Patients may present with the following symptoms:
- Visual blurring
- Foggy vision
- Discomfort, pain, itching, foreign body sensation
- Floaters
Clinical diagnosis
The diagnosis is one of exclusion. It should be considered when a patient is seropositive for HTLV-1 with no systemic evidence of HTLV-1-related diseases (such as ATL or HAM/TSP) after exclusion of other potential uveitis causes[17].
Differential diagnosis
The differential diagnosis depends on the ocular presentation but multiple sclerosis, syphilis, sarcoidosis, and Behçet’s disease are among the diagnosis to consider[27].
Management
Medical therapy
Corticosteroid treatment is effective for treating intraocular inflammation in HU. Treatment intensity should reflect the degree of ocular inflammation: mild inflammation can be managed with topical non-steroidal or steroid anti-inflammatory drugs; a sub Tenon corticosteroid injection may be used in moderate vitritis; in severe ocular inflammation, oral corticosteroids should be administered, although long term systemic therapy should be avoided[17].
Prognosis
Most cases respond favourably, however, approximately 60% of patients recur[28].
References
- ↑ Jump up to: 1.0 1.1 1.2 Goncalves D., Proietti F., Ribas J., Araujo M., Pinheiro S., Guedes A., Carneiro-Proietti A., Epidemiology, Treatment, and Prevention of Human T-Cell Leukemia Virus Type 1-Associated Diseases, Clinical Microbiology Reviews, July 2010, vol.23:577-589
- ↑ Cooper, S. A., M. S. Van der Loeff, and G. P. Taylor. The neurology of HTLV-1 infection. Pract. Neurol. 2009. Vol 9:16–26.
- ↑ Jump up to: 3.0 3.1 Pinheiro S., Martins-filho O., Ribas J., Catalan-soares B., Proietti F., Namen-lopes S., Brito-melo G., Carneiro-proietti A., Immunologic Markers, Uveitis, And Keratoconjunctivitis Sicca Associated With Human T-cell Lymphotropic Virus Type 1, American Journal of Ophthalmology, 2006, vol 142:811-815
- ↑ Jump up to: 4.0 4.1 Mochizuki M, Watanabe T, Yamaguchi K, et al. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res 1992; 83: 236–239.
- ↑ Jump up to: 5.0 5.1 Nakao, K., N. Ohba, M. Nakagawa, and M. Osame. 1999. Clinical course of HTLV-I associated uveitis. Jpn. J. Ophthalmol. 43:404–409.
- ↑ Jump up to: 6.0 6.1 6.2 Mochizuki, M., T. Watanabe, K. Yamaguchi, K. Yoshimura, S. Nakashima, M. Shirao, S. Araki, K. Takatsuki, S. Mori, and N. Miyata. Uveitis associated with human T-cell lymphotropic virus type I: seroepidemiologic, clinical and virologic studies. Journal if Infectious Diseases, 1992 vol 166:943–944.
- ↑ Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. U. S. A. 77:7415–7419.
- ↑ Ehrlich, G. D., J. Andrews, M. P. Sherman, S. J. Greenberg, and B. J. Poiesz. 1992. DNA sequence analysis of the gene encoding the HTLV-I p21e transmembrane protein reveals inter- and intra-isolate genetic heterogeneity. Virology 186:619–627
- ↑ de The´, G., and M. Kazanji. 1996. An HTLV-I/II vaccine: from animal models to clinical trials? J. Acquir. Immune Defic. Syndr. Hum. Retrovirol.13:191–198.
- ↑ Jump up to: 10.0 10.1 10.2 Proietti, F. A., A. B. F. Carneiro-Proietti, B. C. Catalan-Soares, and E. L. Murphy. Global epidemiology of HTLV-1 infection and associated diseases. Oncogene, 2005, 24:6058–6068.
- ↑ Murphy, E. L., J. P. Figueroa, W. N. Gibbs, M. Holding-Cobham, B. Cranston, K. Malley, A. J. Bodner, S. S. Alexander, and W. A. Blattner. 1991. Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I. Demographic determinants. Am. J. Epidemiol. 133:1114–1124
- ↑ Ikeda, E., Ono, A., Hikita, N., et al. Estimated prevalence rate of HTLV-I uveitis in Chikugo. J. Jpn. Ophthalmol. 1998Soc. 102:327–332,
- ↑ Osame, M., Janssen, R., Kubota, H., et al. Nationwide survey of HTLV-I-associated myelopathy in Japan: association with blood transfusion. Ann. Neurol. 1990, 28:50–56,
- ↑ Biggar, R. J., J. Ng, N. Kim, M. Hisada, H. C. Li, B. Cranston, B. Hanchard, and E. M. Maloney. 2006. Human leukocyte antigen concordance and the transmission risk via breast-feeding of human T cell lymphotropic virus type I. J. Infect. Dis. 193:277–282.
- ↑ Fujito T, and Y. Nagata. 2000. HTLV-I transmission from mother to child. J. Reprod. Immunol. 47:197–206
- ↑ Sandler SG, Fang C, Williams A. Retroviral infections transmitted by blood transfusion. Yale J Biol Med, 1990vol 63:353-360
- ↑ Jump up to: 17.0 17.1 17.2 Kamoi K, Mochizuki M., HTLV-1 uveitis. Front Microbiol. 2012, vol 3:270
- ↑ Jump up to: 18.0 18.1 Ono,A.,Mochizuki,M.,Yamaguchi,K., Miyata,N., Watanabe,T.Immunologic and virologic characterization of the primary infiltrating cells in the aqueous humor of human T-cell leukemia virus type-1 uveitis. Accumulation of the human T-cell leukemia virus type-1-infected cells and constitutive expression of viral and interleukin-6 messenger ribonucleic acids. Invest.Ophthalmol.Vis. Sci., 1997, 38, 676–689.
- ↑ Sagawa,K.,Mochizuki,M., Masuoka, K., Katagiri,K.,Katayama,T., Maeda,T., Tanimoto,A.,Sugita,S., Watanabe,T.,Itoh,K.Immunopathological mechanisms of human T cell lymphotropic virus type 1 (HTLV-I) uveitis. Detection of HTLV-I-infected T cells in the eye and their constitutive cytokine production. J. Clin.Invest. 1995, vol 95, 852–858.
- ↑ Ono, A., Mochizuki,M., Yamaguchi, K., Miyata,N.,Watanabe,T.. Increased number of circulating HTLV-1 infected cells in peripheral blood mononuclear cells of HTLV-1 uveitis patients: a quantitative polymerase chain reaction study. Br.J.Ophthalmol. 1995, 79, 270–276.
- ↑ Ono,A.,Ikeda,E.,Mochizuki,M.,Matsuoka, M.,Yamaguchi,K.,Sawada, T.,Yamane,S.,Tokudome,S.,and Watanabe,T.Provirus load in patients with human T-cell leukemia virus type 1 uveitis correlates with precedent Graves’ disease and disease activities. Jpn.J.CancerRes. 1998, 89, 608–614.
- ↑ Carneiro-Proietti, A. B. F., B. C. Catalan-Soares, and F. A. Proietti. Human T-cell lymphotropic viruses (HTLV-I/II) in South America: should it be a public health concern?, J. Biomed. Sci. 2002, 9:587–595.
- ↑ Ohba, N.,Matsumoto,M., Sameshima, M., Kabayama,Y., Nakao,K., Unoki,K., Uehara,F., Kawano,K., Maruyama,I., Osame,M. Ocular manifestations in patients infected with human T-lymphotropic virus type I. Jpn.J.Ophthalmol. 1989, 33, 1–12.
- ↑ Terada Y, Kamoi, K, Komizo T, Miyata K,Mochizuki M. Human T-Cell Leukemia Virus Type 1 and Eye Diseases, Journal Of Ocular Pharmacology And Therapeutics, 2017
- ↑ Yamaguchi, K., Mochizuki,M.,Watanabe, T.,Yoshimura,K., Shirao,M., Araki,S., Miyata,N., Mori,S., Kiyokawa,T., Takatsuki,K. Human T lymphotropic virus type 1 uveitis after Graves’ disease. Br. J. Ophthalmol. 1994, 78, 163–166.
- ↑ Jump up to: 26.0 26.1 Buggage RR, Levy-Clarke GA, Smith JA. New corneal findings in human T-cell lymphotrophic virus type 1 infection. Am J Ophthalmol 2001; 132: 950–951.
- ↑ Yanoff M, Duker JS, Ophthalmology. 4th ed., Elsevier Inc., 2014
- ↑ Yoshimura,K., Mochizuki,M., Araki, S., Miyata,N., Yamaguchi,K., Tajima, K., Watanabe,T.Clinical and immunologic features of human T-cell lymphotropic virus type I uveitis. Am.J.Ophthalmol. 1993, 116, 156–163