Home Tonometry
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Home Tonometry Overview[1][2]
Although rebound tonometry has been described as early as the 1930s[1], home tonometry devices have only recently been developed and approved as an alternative for Goldmann applanation tonometry (GAT). The currently available clinical devices for home monitoring of intraocular pressure, iCare HOME rebound tonometer (iCare Finland Oy, Finland) and Triggerfish contact lens sensor (Sensimed), provide different and valuable data for management of patients with glaucoma.
Background[3][4][5][6]
Primary open angle glaucoma (POAG) is one of the major causes of blindness in developed countries.[3] Intraocular pressure (IOP) is the main modifiable risk factor to prevent vision loss due to glaucomatous optic neuropathy.[4] Recent studies have demonstrated nocturnal and diurnal patterns of IOP fluctuation throughout the course of each day[7] which can peak outside of clinic hours.[6][8] Even in eyes without glaucoma, IOP may range 4-5 mmHg throughout the day with a range even wider in glaucomatous eyes. Overnight there is a reproducible rise in IOP related to supine positioning during sleep that is missed with office IOP measurements taken in the upright position. Currently, patients with glaucoma receive infrequent IOP measurements 3-4 times per year at standard of care clinic visits. These IOP measurements, taken only during clinic hours, provide an incomplete clinical assessment of IOP and may inadequately assess disease treatment and prognosis for patients with glaucoma. To more completely assess patient IOP and determine the best course for managing these patients, there is an increased need for daytime or 24-hour phasing to measure IOP readings outside of clinic hours.
Rebound Tonometry[1]
Rebound tonometry, also known as ‘dynamic tonometry,’ has undergone several iterations since its initial conception by Obbink & Weigersma, however the core mechanics behind rebound tonometry remain the same. A probe is accelerated towards the cornea repeatedly and the resultant deceleration, after the probe strikes the cornea and is accelerating away from the cornea, is used to measure the intraocular pressure. An elevated IOP creates a firmer surface for the probe to strike, which results in decreased contact time between the probe and the cornea, as well as a higher acceleration away from the cornea after impact. The amount of rebound acceleration is used to calculate IOP.
While the principle of rebound tonometry was described in 1931, modern rebound tonometry did not reappear as a viable clinical solution until 65 years later when described by Kontiola in 1997.[9] Ten years later in 2007, the Food and Drug Administration approved the iCare Tonometer as the first commercial device approved for rebound tonometry in clinical settings. The iCare Tonometer devices utilizes a 40mm metal probe with a 1.7mm diameter plastic end-tip and is accelerated through a solenoid chamber, when taking a reading. Due to the small size of the probe and brief time of contact, no anesthesia is required to record an IOP measurement. Studies comparing rebound tonometry with Goldmann applanation demonstrate strong agreement between devices of IOP measurements taken with rebound tonometry, remaining consistently within 5 mmHg of GAT.[10][11][12][13]
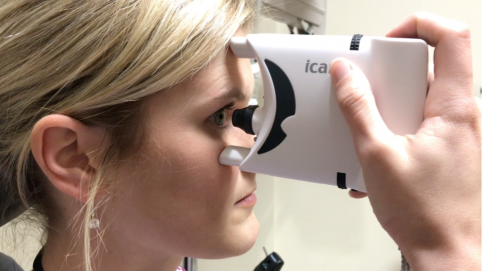
Home Rebound Tonometry: iCare HOME
The iCare HOME (iCare Finland Oy, Finland), approved by the FDA in 2017, is designed for self-tonometry. Once trained on the device, patients are able to take multiple daytime measurements of IOP in an ambulatory setting outside of the clinic.[14] As a rebound tonometer, the iCare HOME functions through the same mechanism of the clinical version of the iCare tonometer where a small metal probe with a plastic tip is suspended in a solenoid chamber. When a reading is initiated, the probe is accelerated towards the cornea, and the rate of deceleration of the probe is used to determine an intraocular pressure reading in millimeters of mercury. Several unique features of the iCare HOME allow for self tonometry at home. These include its compact size, the addition a light ring around the probe chamber to guide centering of the probe, and adjustable forehead/cheek supports.
To use the iCare HOME, patients power on the device using the power button on the back of the device. After pressing the top button, a new probe is loaded with the metal end entering the chamber and plastic tip facing externally. Pressing the top button once more activates the solenoid chamber and locks the probe into place, readying the device for use. The patient lines the green/red indicator ring 4-8 millimeters from their cornea. Adjustable forehead and cheek rests are used to accurately position the probe away from the cornea with unique settings for each eye. When the device is parallel to the ground, the circular light will glow green indicating a measurement may be taken. Pushing the top button again will accelerate the probe towards the cornea. Six consecutive measurements are averaged together to create one IOP reading. Each IOP measurement is saved along with the time, date, and eye of the reading.
Use of the device for self-tonometry outside of standard clinic hours may capture diurnal fluctuations in IOP missed by in office readings and provide more complete image of a patient’s overall IOP circadian curve[7] and is a portable tool for home tonometry, as the iCare HOME does not require use of topical anesthetic for measurement. Studies have demonstrated a strong correlation between the iCare HOME and GAT with inter-device agreement within 5 mmHg.[11][15][16][17]
Despite its advantages, not everyone may be able to accurately use the device for self-tonometry. Some previous studies have reported 16-25% of patients having difficulties completing training.[5][7][11][16] There are also conflicting reports which note a tendency for the iCare HOME to underestimate[17][18] or overestimate[15] IOP when directly compared to GAT. Finally due to reliance on a self-administered tonometry session, the iCare HOME may miss nocturnal IOP spikes.[2]
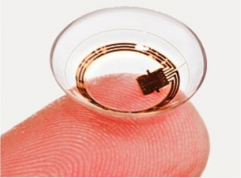
Triggerfish Contact Lens Sensor[2][3]
In 2016, the FDA approved the Triggerfish contact lens sensor (CLS, Sensimed, Lausanne, Switzerland), which was designed for non-invasive, continuous ocular telemetry. The Triggerfish Sensor is manufactured as a single-use silicone contact lens which comes in three sizes based on base curve: steep (8.4 mm), medium (8.7 mm) and flat (9.00 mm). Using embedded strain gauges and a microchip, the CLS measures changes in corneoscleral shape as a surrogate for IOP measurement. Data are transmitted wirelessly to an antenna fixated to the periorbital region. Data are stored in a portable recorder worn by the patient and transferred via Bluetooth to a provider’s computer.[19] The CLS is designed to be worn for a continuous 24-hour period to determine IOP fluctuations in an ambulatory setting.[2][20]
The strain gauge records 300 IOP readings over a 30-second interval every 5 minutes, resulting in 86,400 data points collected in a 24-hour period. Of note, the Triggerfish CLS collects readings in millivolt equivalents (mVeq) instead of the clinical standard of millimeters of mercury (mmHg). The CLS measures the degree of corneoscleral deformation secondary to volume changes as an indirect measure of IOP. A conversion of mVeq to mmHg is not possible due to the viscoelastic properties of the eye and the non-linear relationship of volume and pressure.[2]
While mild adverse events are commonly reported with use of Triggerfish CLS, no severe adverse effects have been reported. The most frequent adverse events associated with Triggerfish CLS use include transient blurred vision, conjunctival hyperemia, superficial punctate keratitis.[20] All of these resolve with discontinuation of device wear.
Preliminary studies of Triggerfish show fair to good reproducibility.[20][21][22] A study by Mansouri and colleagues compared two 24-hour CLS recordings in 40 patients spaced one week apart, demonstrated fair to good correlation between the two intervals (r=.59),[21] and a different study of 9 patients by Hollò et al. found a strong correlation (r=.733) between baseline CLS curves measured four days apart.[22] However, the CLS’ validity has not yet been fully elucidated. Due to its potential for non-invasive nocturnal monitoring, several groups have utilized Triggerfish CLS to study circadian patterns of IOP with mixed results. Studies which have stratified glaucoma patients by subset of disease (pseudoexfoliative glaucoma, primary open angle closure glaucoma, normal tension glaucoma), have demonstrated Triggerfish CLS’ ability to detect nocturnal peaks in IOP.[20] The CLS has been able to demonstrate significant reductions of nocturnal IOP spikes after selective laser trabeculoplasty treatment. However, a study by Holló and colleagues using the CLS to detect IOP reductions from prostaglandin analogue therapy found no difference in mean signal from Triggerfish profiles before and after treatment. They also noted a continuous time-dependent increase in IOP for all CLS measurements during the first and last 50 minutes of CLS wear. This study was also unable to detect the widely accepted IOP change due to posture (due to laying supine for sleep or sitting up upon waking).[22] Nonetheless, the generated 24-hour profiles can determine when peak IOP levels may occur for office visit timing. There is also evidence that IOP profile parameters may be predictive of fast vs slow progressing disease.[23] Despite the promise and novelty of the device, more research is needed before Triggerfish profiles can be more completely integrated into clinical practice.
EyeMate
EyeMate is a permanent, implantable IOP sensor, developed by German company Implandata Ophthalmic Products, which is inserted into the ciliary sulcus behind the iris and in front of an artificial lens during cataract surgery. The device consists of eight pressure- and temperature-sensitive capacitors attached to a gold circular antenna, and readings are transmitted through an external handheld device which also charges the Eyemate through electromagnetic coupling.[24][25] This allows for permanent on-demand IOP readings that may be taken by patients at any time in an ambulatory setting.
The first long-term study of patients implanted with the EyeMate, the ARGOS-1 study, did not find any severe adverse events after implantation with Eyemate,[24] although variable pupillary distortion was noted in all six patients of this study. Koutsounas et al. also noted “varying degrees of bilateral deterioration of visual fields” for all patients, but attributed this to progression of glaucoma.[24] Further studies are needed to determine the safety and utility of Eyemate in long-term glaucoma monitoring. The Eyemate has reached CE mark in Europe, but is not approved by the United States FDA.
Summary Table
| iCare HOME | Triggerfish | Eyemate | |
|---|---|---|---|
| Advantages |
|
|
|
| Disadvantages |
|
|
|
References
- ↑ Jump up to: 1.0 1.1 1.2 Dekking HM, Coster HD. Dynamic tonometry. Ophthalmologica. 1967;154(1):59-74.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Young CC, Seibold LK. 24-hour IOP monitoring: Current state and future directions. Glaucoma Physician. 2018:10-14.
- ↑ Jump up to: 3.0 3.1 3.2 Aptel F, Musson C, Zhou T, Lesoin A, Chiquet C. 24-hour intraocular pressure rhythm in patients with untreated primary open angle glaucoma and effects of selective laser trabeculoplasty. J Glaucoma. 2017;26(3):272-277.
- ↑ Jump up to: 4.0 4.1 Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53 Suppl1:S3-10.
- ↑ Jump up to: 5.0 5.1 Meier-Gibbons F, Berlin MS, Toteberg-Harms M. Twenty-four hour intraocular pressure measurements and home tonometry. Curr Opin Ophthalmol. 2018;29(2):111-115.
- ↑ Jump up to: 6.0 6.1 Arora T, Bali SJ, Arora V, Wadhwani M, Panda A, Dada T. Diurnal versus office-hour intraocular pressure fluctuation in primary adult onset glaucoma. J Optom. 2015;8(4):239-243.
- ↑ Jump up to: 7.0 7.1 7.2 Huang J, Katalinic P, Kalloniatis M, Hennessy MP, Zangerl B. Diurnal intraocular pressure fluctuations with self-tonometry in glaucoma patients and suspects: A clinical trial. Optom Vis Sci. 2018;95(2):88-95.
- ↑ Moon Y, Lee YL, Jeong DW, Kim S, Han S, Kook MS. Relationship between nocturnal intraocular pressure elevation and diurnal intraocular pressure level in normal-tension glaucoma patients. IOVS. 2015;56(9):5271-5279.
- ↑ Kontiola A. A new electromechanical method for measuring intraocular pressure. Doc Ophthalmol. 1996;93(3):265-276.
- ↑ Gandhi NG, Prakalapakorn SG, El-Dairi MA, Jones SK, Freedman SF. Icare one rebound versus Goldmann Applanation Tonometry in children with known or suspected glaucoma. Am J Ophthalmol. 2012;154(5):843-849.e841.
- ↑ Jump up to: 11.0 11.1 11.2 Mudie LI, LaBarre S, Varadaraj V, et al. The icare home (ta022) study: Performance of an intraocular pressure measuring device for self-tonometry by glaucoma patients. Ophthalmology. 2016;123(8):1675-1684.
- ↑ Pronin S, Brown L, Megaw R, Tatham AJ. Measurement of intraocular pressure by patients with glaucoma. JAMA Ophthalmol. 2017;135(10):1-7.
- ↑ Kato Y, Nakakura S, Matsuo N, et al. Agreement among Goldmann Applanation Tonometer, iCare, and iCare Pro rebound tonometers; non-contact tonometer; and tonopen xl in healthy elderly subjects. Int Ophthalmol. 2018;38(2):687-696.
- ↑ Icare home tonometer 501(k). In:2017.
- ↑ Jump up to: 15.0 15.1 Takagi D, Sawada A, Yamamoto T. Evaluation of a new rebound self-tonometer, icare home: Comparison with Goldmann Applanation Tonometer. J Glaucoma. 2017;26(7):613-618.
- ↑ Jump up to: 16.0 16.1 Dabasia PL, Lawrenson JG, Murdoch IE. Evaluation of a new rebound tonometer for self-measurement of intraocular pressure. Br J Ophthalmol. 2016;100(8):1139-1143.
- ↑ Jump up to: 17.0 17.1 Noguchi A, Nakakura S, Fujio Y, et al. A pilot evaluation assessing the ease of use and accuracy of the new self/home-tonometer iCare HOME in healthy young subjects. J Glaucoma. 2016;25(10):835-841.
- ↑ Termuhlen J, Mihailovic N, Alnawaiseh M, Dietlein TS, Rosentreter A. Accuracy of measurements with the iCare HOME rebound tonometer. J Glaucoma. 2016;25(6):533-538.
- ↑ Sensimed. About sensimed triggerfish. https://www.sensimed.ch/sensimed-triggerfish/. Accessed November 11, 2018.
- ↑ Jump up to: 20.0 20.1 20.2 20.3 20.4 Dunbar GE, Shen BY, Aref AA. The sensimed triggerfish contact lens sensor: Efficacy, safety, and patient perspectives. Clin Ophthalmol. 2017;11:875-882.
- ↑ Jump up to: 21.0 21.1 Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: Safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol. 2012;130(12):1534-1539.
- ↑ Jump up to: 22.0 22.1 22.2 Hollo G, Kothy P, Vargha P. Evaluation of continuous 24-hour intraocular pressure monitoring for assessment of prostaglandin-induced pressure reduction in glaucoma. J Glaucoma. 2014;23(1):e6-12.
- ↑ De Moraes CG, Mansouri K, Liebmann JM, Ritch R. Association between 24-hour intraocular pressure monitored with contact lens sensor and visual field progression in older adults with glaucoma. JAMA Ophthalmol. 2018;136(7):779-785.
- ↑ Jump up to: 24.0 24.1 24.2 Koutsonas A, Walter P, Roessler G, Plange N. Long-term follow-up after implantation of a telemetric intraocular pressure sensor in patients with glaucoma: A safety report. Clin Exp Ophthalmol. 2018;46(5):473-479.
- ↑ Ittoop SM, SooHoo JR, Seibold LK, Mansouri K, Kahook MY. Systematic review of current devices for 24-h intraocular pressure monitoring. Adv Ther. 2016;33(10):1679-1690.
- ↑ Querat L, Chen E. Monitoring daily intraocular pressure fluctuations with self-tonometry in healthy subjects. Acta Ophthalmol. 2017;95(5):525-529.