High-Intensity Focused Ultrasound Circular Cyclophotocoagulation
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Glaucoma is a worldwide disease burden for which new effective and cost-effective treatments are needed. Elevated intra-ocular pressure (IOP) is considered the greatest risk factor for glaucoma development and progression, and lowering IOP is still the only consistently proven approach for halting disease progression and loss of vision.[1][2] IOP-lowering drugs, light amplification by stimulated emission of radiation (LASER), and surgery are treatment alternatives. However, treatment of refractory glaucoma represents a clinical challenge.
It has been known since the 1860s that cyclodialysis induced eye globe hypotension. In the 1930s, Vogt et al. first described the destruction of the ciliary body as an option to reduce IOP in refractory glaucoma.[3] Since then, many strategies to destroy the ciliary body – termed cyclodestructive, or cycloablative procedures – have been proposed, including cyclodiathermy, cycloelectrolysis, cyclocryotherapy, and laser cyclophotocoagulation (continuous-wave transscleral LASER, micropulse transscleral LASER, endocyclophotocoagulation (ECP), ECP-plus).
Cyclodestructive procedures are established treatment methods for refractory glaucoma, and diode transscleral Cyclophotocoagulation is currently regarded as the clinical standard.[4] However, these methods have two major drawbacks that have limited their use in earlier cases of glaucoma. Most options are non-selective for the target tissue, causing damage to adjacent structures. Additionally, they have an unpredictable dose-effect relationship that has prevented accurate prediction of the effect of treatment.[1][2][4] To overcome these limitations, ultrasound cyclocoagulation has arisen as a potentially safer, more selective and precise approach to destroy the ciliary body tissue and reduce IOP.
High-Intensity Focused Ultrasound (HIFU) systems in the treatment of glaucoma
HIFU technology was tested in the past for treatment of other ophthalmic conditions, including cataract, vitreoretinal, and choroidal lesions. In 1985, Coleman et al. first described the use of high-intensity focused ultrasound for cyclodestruction.[5][6] HIFU cyclocoagulation has proven to an effective and well-tolerated method for reducing the IOP. Studies reported 38.4-42.2% reduction in IOP for months after the first procedure in eyes with refractory glaucoma; IOP below 25mmHg was reported in up to 90% of eyes at 3-month follow-up, and in 65% at 1 year.[7][8][9]
Advantages of the ultrasound over the laser is that the energy can be focused through non-optically transparent media without controlled energy absorption, which reduces the collateral effects in adjacent tissues. Focused ultrasound, unlike diode laser, can be used to heat and treat a defined and adjustable tissue volume at any depth or location within the eye. However, HIFU was abandoned in the 1990s, in part because of the bulky design of the commercially available system (Therapeutic Ultrasound System, Sonocare, Inc., Ridgewood, NJ), and in part because of the complexity of the procedure and time required for the procedure (which could take up to 2 hours in the operating theater). Additionally, the relatively low frequency of the device (5 MHz) created a wide focal zone, potentially injuring neighboring tissue. Most importantly, severe complications posed a significant risk, including scleral staphyloma, corneal thinning, persistent hypotony, phthisis bulbi¸ and vision loss.
Miniaturized HIFU Ultrasound Circular Cyclocoagulation
In 2010, Aptel et al. described the use a new HIFU production system using miniaturized transducers to induce cyclocoagulation,[4] coined ultrasound circular cyclocoagulation (abbreviated as UCCC, or UC3). With miniaturized transducers, ultrasound focusing is better controlled, creating small focal ones that better target the treatment areas. Complex transducers allow for lesions of variable geometries adapted to organs with complex anatomy, such as the ciliary body. In addition, a higher operating frequency allows for a steeper transition between the focal treatment zone and the untreated areas, minimizing risk of heating adjacent ocular tissue. Three-dimensional (3D) reconstruction of the tissue allows for better planning and reduced energy delivery to the tissue.
The cyclocoagulation device EyeOP1 HIFU (EyeTechCare, Rillieux-la-Pape, France) used in UCCC is an automated, computer-assisted, non-operator-dependent cyclodestructive procedure that utilizes a circular-shaped probe matching the 3D anatomy of the ciliary body, which allows correctly focusing the target tissue.[10] The treatment device consists of a therapeutic circular probe, a polymer coupling cone, a touch screen console, and a dual-function foot pedal which allows activation of the treatment (suction and firing phases). The circular probe is connected to the console by an electric cable; the coupling cone is connected to the console by a tube. The probe is a 30 mm diameter, 15 mm high ring divided in 6 cylindrical piezoceramic transducers which generate six ultrasound beams, distributed equidistantly between them (3 in the superior and 3 in the inferior regions) that allow treatment of up to 45% of the ciliary body using the new-generation probes.[2] The target zone is focused with high precision, not exceeding 0.1 x 1 mm in size.
Transducers in miniaturized HIFU are operated at a higher frequency (21 MHz) than the previous HIFU, with an acoustic power of 2.0-2.45 watts (W). Probes are manufactured in three different sizes (11, 12, and 13 mm) in order to fit most ocular sizes; nanophthalmic and megalophthalmic eyes are exceptions. Probe sizes is determined pre-operatively based on ultrasound biomicroscopy (UBM) biometric data to determine the locations of the focal zones.
Mechanisms of action
Cyclodestructive procedures are thought to decrease IOP by reducing aqueous humor (AH) synthesis by destroying the ciliary body epithelium. More recently, other mechanisms have been proposed, namely increased AH outflow via the uveoscleral pathway.
Ultrasound produces thermal increase in tissues, up to 80 ºC, which leads to coagulation necrosis of tissues. Pathology studies in animals[4] proved that miniaturized HIFU produced effective ciliary body destruction, inducing coagulation necrosis at the most intermediate and distal parts of the ciliary processes with loss of the bilayered epithelium, edema, and vascular congestion; the epithelium seems to be preserved in the basal part of the ciliary processes, and the stroma shows no fibrosis. Little inflammation of the ciliary processes and other ocular structures occurs. The treated areas were very precisely delineated from the non-treated areas. The pilot study in humans showed cystic involution of the ciliary body using UBM, with multiple cystic cavities.[11] The extent of destruction of the ciliary processes is correlated to the treatment dosage, with a treatment volume of 4.8 mm3 with the 4-second protocol versus a volume of 7.8 mm3 with the 6-second protocol.[2]
An increase in the suprachoroidal and transscleral portions of the uveoscleral pathway have also been hypothesized to contribute to IOP reduction after ultrasound cyclocoagulation. An in vivo human study using UBM showed the formation of suprachoroidal fluid spaces in 8 of 12 treated eyes, which correlated with lower IOP.[11] Using anterior segment optical coherence tomography (AS-OCT) Mastropasqua et al.[12] documented the formation of new intrascleral hyporeflective spaces (HS), or enlargement of previous HS. They suggested the formation of theses HS would be the consequence of a thermic-induced scleral fiber delamination; this would result from the generation of a temperature gradient between the ciliary body and the ocular surface, which is exposed to a thermic halo produced by the transducer at the site of insonification. In addition, this group showed an increase of conjunctival microcysts at the site of insonification using in vivo confocal microscopy, hypothesized to be a hallmark of the passage of the AH through the sclera and finally the conjunctiva.[12]
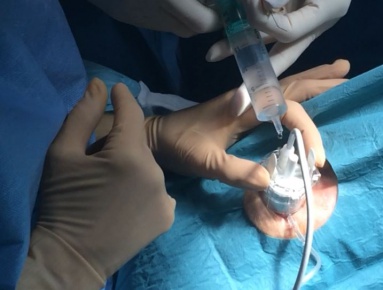
Surgical Technique
The procedure can be performed under topical, peribulbar, or general anesthesia, although most cases are amenable to local anesthesia.
The coupling cone is placed in direct contact with the ocular surface, allowing optimal positioning of the probe in terms of centering and distance, and a stable alignment in terms of the optical axis. To selectively impact with the ciliary body, the ultrasound beam is focused at a depth of 2 mm below the sclera, which would correspond to the spatial position of the ciliary body.[2] The coupling cone is connected to a suction ring, producing a low-level vacuum (70 mmHg) to maintain the cone in contact with the eye during the procedure without movement and misalignment. The space between the eye, the coupling cone, and the probe is filled with approximately 4 mL of room-temperature balanced salted-solution (BSS) to ensure acoustic propagation.[11]
Transducers are sequentially activated clockwise, starting from the superior sectors. Activation of each transducer can last 4, 6, or 8 seconds, with 20 seconds of interval between each sector treatment; the transition between each sector is completely automatic.
In order to be safe and efficient, the system respects four anatomical constraints: avoiding insonification of the cornea and lens, avoiding the nasal and temporal zones during treatment, minimizing the distance between tissues, and avoiding retinal overexposure.[2]
Post-procedural medications include flurbiprofen, or a combination of dexamethasone and tobramycin four times daily for one month.
Outcomes
The first clinical pilot study using miniaturized HIFU in refractory glaucoma was conducted by Aptel et al in 2011.[11] They documented a surgical success rate of 83.3 % (IOP reduction of at least 20% and IOP > 5 mmHg), a significant reduction in IOP from a mean pre-operative value of 37.9 +/- 10.7 mmHg to 26.3 +/ 5.1 mmHg at 3-month follow-up (mean IOP reduction of 35.7 %), with no major intra-operative or postoperative complications. . The EyeMUST1 Study was a 12-month open-label multicenter prospective study conducted to determine the efficacy and safety of UCCC.[13] Primary outcome was IOP reduction at 12 months; secondary endpoints were intra- and postoperative tolerance and visual acuity, IOP lowering medications, and complication rates. Success (defined as an IOP decrease > 20 % and IOP > 5 mmHg) was achieved in 57.1 % of the patients; success was lower in patients with secondary glaucoma compared to patients with primary open-angle glaucoma (45.0 vs 78.6 %). Mean IOP reduction at 6 months was 30.2 %, and at 1 year was 36.0 %. However, this study found no reduction in IOP-lowering medications.
Other studies have been conducted to evaluate the clinical outcomes of UCCC in refractory glaucoma.[1][12][14] Overall, it seems that UCCC tends to have lasting efficacy in controlling the IOP during the first year, with a success rate ranging from 48% to 83.3%.[2]
Whether duration of exposure influences treatment efficacy remains controversial. The pilot study by Aptel et al.[11] showed IOP reduction was significantly greater in the patient group treated with the higher dose. The studies by Mastropasqua et al.[12] and Giannacare et al.[1] also suggested duration of treatment correlated with outcomes. However, the EyeMUST1 study showed no statistically significant differences between patients treated with the 4-second dose and patients treated with the 6-second dose.[13]
Despite these initial studies, more recent prospective trials point to better outcomes. Giannacare et al.[1] also designed a 12 month prospective trial with two groups submitted to UCCC procedures with first (6s) or second (8s) generation probes. This trial registered a complete success (IOP reduction of at least 20% and IOP between 5 and 21 mmHg without any IOP-lowering medications) in 42.9% and a qualified success (IOP reduction of at least 20% and IOP >5 mmHg without any IOP-lowering medications) in 51.1% patients. At 12 months, mean IOP reduction was of 28.7% (from 27.7+-9.2 to 19.8+-6.9 mmHg) with a significant improvement in IOP decrease when comparing first and second generation probes (25.6% vs. 35%, respectively). The IOP lowering average values did not differ significantly according to types of glaucoma and to lens status. This study also found a significant linear relation between preoperative IOP and its decrease after surgery, with greater IOP reductions in patients with greater preoperative IOP values. This relation permits UCCC to be effective in patients with highly elevated IOP, while at the same time, remaining safe to patients with lower baseline IOP values by reducing or eliminating the risk of postoperative hypotony. There was a significant decrease in the average number of IOP lowering medications at 12 months (from 3.2 to 2.3). This study did not report any major intra- or postoperative complication during the 12 month follow-up period.
De Gregorio et al.[15] designed another 12 month prospective trial with the possibility to repeat 2nd generation probe (8s) UCCC procedures with a minimum interval of 4 months(needed if IOP had risen above 21 mmHg with maximum therapy) having registered a 85% surgical success (IOP between 5-21 mmHg) without any IOP-lowering medications at 12 months. The repeated procedures were realised without rotating the probe due to the hypothesis of possible epithelium regeneration and reactivation of the ciliary body. In this trial, 45% patients needed 1 procedure, 50% needed 2 procedures and 30% needed 3 procedures, registering an average of 2.35 procedures per patient. The significant reductions in mean IOP at 12 months after first procedure were of 45.7%, 32.4% and 36.5% (1 procedure, 2 procedures and 3 procedures, respectively) without any major intra-operative or postoperative complications. There was a significant decrease in the average number of IOP lowering medications at 12 months (from 3.6+-0.7 to 2.4+-1.3).
Complications
The findings by clinical studies suggest miniaturized HIFU UCCC as a good safety profile, with low rates of intra- and postoperative complications. Complications described for UCCC include:[2][11][13]
- Intraoperative pain
- Intraoperative subconjunctival hemorrhage
- Conjunctival hyperemia
- Superficial punctate keratitis
- IOP spikes (above 8 mmHg from baseline)
- Anterior chamber reaction
- Focal scleral thinning
- Transient hypotony with/without choroidal detachment
- Corneal edema
- Transient macular edema
- Focal scleral thinning
The EyeMUST1 Study[13] documented few intra-operative complications associated with UCCC: 4 out of the 52 patients reported tolerable, transient pain during the procedure; 1 patient had an IOP spike which resolved within 1 hour of instillation of apraclonidine; and 2 patients had subconjunctival hemorrhage. Postoperatively, 33% of patients had superficial punctate keratitis, 48% reported conjunctival hyperemia, 13 patients had transient anterior chamber uveal reaction, and 7.7% had transient corneal edema; 1 patient showed postoperative hypotony with choroidal detachment which resolved within 1 month of topical steroid treatment; and 2 patients had macular edema which resolved after 1 month of non-steroidal anti-inflammatory agents. 6 patients had vision loss of more than two lines. No significant differences were observed regarding the mean number of IOP lowering drugs. 12 patients required secondary glaucoma surgical intervention due to insufficient response to the HIFU treatment.
De Gregorio et al.[15] documented similar intra-operative complications associated with UCCC: pain (30/40) and sub-conjunctival haemorrhage (15/40). Regarding post-operative complications, only minor complications were registered, such as conjunctival hyperemia in 40/40 (100%), as superficial punctate keratitis 18/40 (45%) and such as sub-conjunctival hemorrhage 12/40 (30%). No major complications were observed post-procedure, without any case of corneal edema, of choroidal detachment, of phtisis or anterior chamber reaction having been registered.
Conclusions
Cyclodestructive procedures are currently limited to refractory or end-stage glaucoma. The UCCC procedure has shown good efficacy and safety profiles in clinical studies, providing a newer treatment strategy in the treatment of refractory glaucoma, apparently safer than the previous cyclodestructive options. Given these data, Aptel et al. conducted a prospective study to evaluate the efficacy of UCCC in the treatment of patients with early glaucoma naïve of previous filtering surgery.[16] Mean IOP reduction at 12 months was 37%; complete success (defined as IOP reduction > 20% from baseline, IOP > 5 mmHg, and IOP < 21 mmHg without adjuvant medication) was 46.7%; and qualified success (defined as IOP reduction > 20% from baseline and IOP > 5 mmHg without adjuvant medication) was 63%. No major intra- or postoperative complications were reported. Graber et al. conducted a prospective one-armed single center pilot study to evaluate the efficacy and safety of miniaturized HIFU in patients with chronic angle-closure glaucoma.[17] They reported a decrease in IOP from 18.4 +/- 3.5 mmHg preoperatively to 14.8 +/- 4.1 mmHg 6 months after UCCC, with no significant side effects and stable visual acuity.
De Gregorio et al.[15] registered an 85% surgical success rate (IOP between 5-21 mmHg) without any IOP-lowering medications at 12 months and significant reductions in mean IOP of 45.7%, 32.4% and 36.5% (1 procedure, 2 procedures and 3 procedures, respectively) in all types of glaucoma patients (primary open angle, chronic close angle, exfoliative, neovascular and uveitic with similar frequencies) with a ratio of previous filtering surgery to naïve to previous filtering surgery of 1.3;1. Thus, this study supports the previous findings of 12-month efficacy not only in advanced glaucoma patients but also in early glaucoma still naïve to filtering surgery, with no major intra- or postoperative complications reported during the follow-up period.
These findings have made UCCC an appealing treatment option for both refractory and earlier-stage glaucoma, both open-angle and angle-closure. However, the longest follow-up results reported are at 12-month postoperative. This highlights the need for longer, prospective, randomized controlled trials to fully understand the place and timing of this technique in the treatment of glaucoma.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Giannaccare, G., Vagge, A., Gizzi, C. et al. Graefes Arch Clin Exp Ophthalmol (2017) 255: 599. doi:10.1007/s00417-016-3563-z
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Rodolfo Mastropasqua, Vincenzo Fasanella, Alessandra Mastropasqua, Marco Ciancaglini, and Luca Agnifili, “High-Intensity Focused Ultrasound Circular Cyclocoagulation in Glaucoma: A Step Forward for Cyclodestruction?,” Journal of Ophthalmology, vol. 2017, Article ID 7136275, 14 pages, 2017. doi:10.1155/2017/7136275
- ↑ Vogt A. Versuche zur intraokularen druckherabsetzung mittelst diathermiescha¨digung des corpus ciliare Zyklodiathermiestichelung (1936)
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Aptel F, Charrel T, Palazzi X, Chapelon JY, Denis P, Lafon C. Histologic effects of a new device for high-intensity focused ultrasound cyclocoagulation. Invest Ophthalmol Vis Sci. 2010; 51:5092–5098.
- ↑ Coleman DJ, Lizzi FL, Driller J, et al. Therapeutic ultrasound in the treatment of glaucoma. I. Experimental model. Ophthalmology. 1985;92:339–346.
- ↑ Coleman DJ, Lizzi FL, Driller J, et al. Therapeutic ultrasound in the treatment of glaucoma. II. Clinical applications. Ophthalmology. 1985;92:347–353.
- ↑ Burgess SE, Silverman RH, Coleman DJ, et al. Ophthalmology. 1986;93:831–838.
- ↑ Maskin SL, Mandell AI, Smith JA, Wood RC, Terry SA. Therapeutic ultrasound for refractory glaucoma: a three-center study. Ophthalmic Surg. 1989;20:186–192.
- ↑ Sterk CC, van der Valk PH, van Hees CL, van Delft JL, van Best JA, Oosterhuis JA. The effect of therapeutic ultrasound on the average of multiple intraocular pressures throughout the day in therapy resistant glaucoma. Graefes Arch Clin Exp Ophthalmol. 1989;227:36–38.
- ↑ Charrel T, Aptel F, Birer A, Chavrier F, Romano F, Chapelon JY et al (2011) Development of a miniaturized HIFU device for glaucoma treatment with conformal coagulation of the ciliary bodies. Ultrasound Med Bi o l 37:742–754. doi :10.1016/j.ultrasmedbio.2011.01.017
- ↑ Jump up to: 11.0 11.1 11.2 11.3 11.4 11.5 Aptel F, Charrel T, Lafon C, Romano F, Chapelon JY, Blumen-Ohana E et al (2011) Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci 52:8747–8753. doi:10.1167/iovs.11-8137
- ↑ Jump up to: 12.0 12.1 12.2 12.3 Mastropasqua R, Agnifili L, Fasanella V, Toto L, Brescia L, Di Staso S et al (2016) Uveo-scleral outflow pathways after ultrasonic cyclocoagulation in refractory glaucoma: an anterior segment optical coherence tomography and in vivo confocal study. Br J Ophthalmol. doi:10.1136/bjophthalmol-2015-308069
- ↑ Jump up to: 13.0 13.1 13.2 13.3 Denis P, Aptel F, Rouland J-F, et al. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: a 12-month multicenter study. Invest Ophthalmol Vis Sci. 2015;56:1089–1096. DOI:10.1167/iovs.14-14973
- ↑ Aptel F, Dupuy C, Rouland JF. Treatment of refractory open angle glaucoma using ultrasonic circular cyclocoagulation: a prospective case series. Curr Med Res Opin. 2014;30:1599–1605.
- ↑ Jump up to: 15.0 15.1 15.2 De Gregorio A, Pedrotti E, Stevan G, Montali M, Morselli S. Safety andefficacy of multiple cyclocoagulation of ciliary bodies by high-intensity focused ultrasound in patients with glaucoma. Graefes Arch ClinExpOphthalmol. 2017Dec;255(12):2429-2435. doi: 10.1007/s00417-017-3817-4. Epub 2017 Oct 17.
- ↑ F. Aptel, P. Denis, J. F. Rouland, J. P. Renard, and A. Bron, “Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery,” Acta Ophthalmologica, vol. 94, no. 5, pp. e268–e277, 2016.
- ↑ Graber M1, Khoueir Z2, Beauchet A2, Benhatchi N2, Hammoud S2, Lachkar Y2. High intensity focused ultrasound as first line treatment in patients with chronic angle closure glaucoma at risk for malignant glaucoma. J Fr Ophtalmol. 2017 Apr;40(4):264-269. doi: 10.1016/j.jfo.2016.10.013. Epub 2017 Mar 23.

