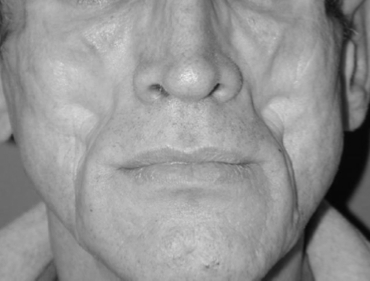
HIV-Associated Facial Lipoatrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
HIV-associated lipoatrophy, which includes fat loss of the limbs, buttocks, and face, has been closely linked to the use of the thymidine nucleoside reverse-transcriptase inhibitors stavudine and zidovudine. The lipoatrophy can have severe psychological effects and is also associated with increased risk of metabolic disorders and cardiovascular disease. Cessation of the causative drugs usually does not result in noticeable improvement of the lipoatrophy.
Disease Entity
Disease
A syndrome of peripheral lipoatrophy affecting HIV-infected patients was first described in 1998, coinciding with the introduction of highly active antiretroviral treatment (HAART) [1]. Fat is lost from the limbs, buttocks, and face [2].
Etiology
Although initially ascribed to protease inhibitor treatment, further clinical studies indicated that the use of thymidine nucleoside reverse-transcriptase inhibitors (NRTIs), particularly stavudine and zidovudine, is a dominant risk factor. [3] [4] Evidence suggests that the fat wasting is due to NRTI-induced mitochondrial toxicity. [4]
Risk Factors
There is strong associations between stavudine treatment and risk of lipoatrophy. Zidovudine treatment is associated with lipoatrohy to a lesser extent. [4]
General Pathology
Fat is lost from the nasolabial region, the temples and the eye sockets. Of note, lipoatrophy is independently associated with metabolic disorders, including dyslipidemia, insulin resistance, and increased risk of cardiovascular disease. [5] [6] [7]
Pathophysiology
The mitochondrial toxicity associated with stavudine and zidovudine is thought to be related to the medications' inhibitory effect on mitochondrial DNA polymerases. [8] Adipose samples from NRTI-treated patients have less mitochondrial DNA and greater tissue macrophage infiltration as compared to NRTI-naive HIV-positive patients. [4]
Primary Prevention
More severe lipoatrophy is correlatd with cumulative drug exposure [4]. Although there has been a significant reduction in lipoatrophy incidence with the decreasing use of stavudine and zidovudine over the last decade, the persistent nature of established lipoatrophy, with only minimal improvement after switching or removing NRTI drugs [9], has ensured that the syndrome remains prevalent.
Diagnosis
This is a clinical diagnosis.
History
Exposure to stavudine and/or zidovudine. The protease-inhibitors have been implicated to a lesser extent.
Physical Examination
Patients have symmetrical facial fat atrophy.
Signs
N/A
Symptoms
If loss of orbital fat has caused enophthalmos, patients may complain of dry eye symptoms. Facial lipoatrophy can be stigmatizing and has been associated with depression. In one survey of HIV-infected patients, lipoatrophy was associated with higher levels of depression than lipohypertrophy and facial lipoatrophy was associated with the most severe depression scores. [10]
Diagnostic procedures
None
Laboratory test
Because lipoatrophy is independently associated with metabolic disorders, including dyslipidemia, insulin resistance, and increased risk of cardiovascular disease, the patient should be screened for dyslipidemia and diabetes.
Differential diagnosis
N/A
Management
Poly-L-lactic acid and calcium hydroxylapatite are injectable fillers that have been FDA approved for the treatment of facial fat loss in patients with HIV. [8]Additionally, adipose derived progenitor cells, liquid silicone and hyaluronic based fillers have been used to improve cosmesis in this condition. [11][12][13]
Bilateral orbital floor implants can treat enophthalmos.
Prognosis
HIV-associated lipoatrophy has been shown to persist after 5 years of follow up, with little recovery regardless of whether the implicated drugs were discontinued [9]. The use of injectable facial fillers can help patients normalize their appearance.
References
- ↑ Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998; 12:F51-F58.
- ↑ James J, Carruthers A, Carruthers J. HIV-Associated Facial Lipoatrophy. Dermatol Surg 2002; 28:979-986.
- ↑ Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14:1309-16.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Hammond E, McKinnon E, Nolan D. Human Immunodeficiency Virus Treatment-Induced Adipose Tissue Pathology and Lipoatrophy: Prevalence and Metabolic Consequences. HIV/AIDS. 2010;51:591-9.
- ↑ Wohl D, Scherzer R, Heymsfield S, Simberkoff M, Sidney S. The Associations of Regional Adipose Tissue with Lipid and Lipoprotein Levels in HIV-Infected Men. Journal of Acquired Immune Deficiency Syndromes. 2008;48:44-52.
- ↑ De Witt S, Sabin CA, Weber R, Worm SW, Reiss P. Incidence and Risk Factors for New-Onset Diabetes in HIV-infected Patients: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study. Diabetes Care. 2008;31:1224-9.
- ↑ De Socio GV, Parruti G, Quirino T, Ricci E, G S. Identifying HIV patients with an unfavorable cardiovascular risk profile in the clinical practice: results from the SIMONE study. Journal of Infection. 2008;57:33-40.
- ↑ Jump up to: 8.0 8.1 Introcaso CE, Hines JM, Kovarik CL. Cutaneous toxicities of antiretroviral therapy for HIV: Part 1. Lipodystrophy syndrome, nucleoside reverse transcriptase inhibitors, and protease inhibitors. J Am Acad Dermatol. 2010;63:549-561.
- ↑ Jump up to: 9.0 9.1 Grunfeld C, Saag M, Cofrancesco J Jr, et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS 2010;24:1717-26.
- ↑ Crane HM, Grunfeld C, Harrington RD, et al. Lipoatrophy among HIV-infected patients is associated with higher levels of depression than lipohypertrophy. HIV Med 2008;9:780-6.
- ↑ Suzuki K, Akita S, Yoshimoto H, Ohtsuru A, Hirano A, Yamashita S1, Biological Features Implies Potential Use of Autologous Adipose-Derived Stem/Progenitor Cells in Wound Repair and Regenerations for the Patients with Lipodystrophy, Int J Mol Sci. 2019 Nov 5;20(21). pii: E5505. doi: 10.3390/ijms20215505.
- ↑ Jones DH, Carruthers A, Brody HJ, Black JM, Humphrey S, Carruthers J, Wesley NO, Minokadeh A, Ten-Year and Beyond Follow-up After Treatment With Highly Purified Liquid- Injectable Silicone for HIV-Associated Facial Lipoatrophy: A Report of 164 Patients. Dermatol Surg. 2019 Jul;45(7):941-948. doi: 10.1097/DSS.0000000000001889.
- ↑ Hausauer AK, Jones DH. Long-Term Correction of Iatrogenic Lipoatrophy With Volumizing Hyaluronic Acid Filler. Dermatol Surg. 2018 Nov;44 Suppl 1:S60-S62. doi: 10.1097/DSS.0000000000001417.