Gradenigo Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Gradenigo Syndrome (GS) is classically described as a clinical triad of otitis media, facial pain and abducens palsy that is most commonly developed from infection in the petrous temporal bone (i.e., petrous apicitis) [1]. The full triad of GS however may not always be present especially in the post-antibiotic era .
Etiology
GS was first described in 1904 [1] from untreated or partially treated otitis media. In the preantibiotic era, intracranial complications secondary to ear infection occurred in 2.3-6.4% of cases [2], however, with appropriate antibiotic use, it is now increasingly rare [3]. Additionally with the evolution of more refined surgical techniques, intracranial complications decreased to 0.04-0.15% of cases [2].
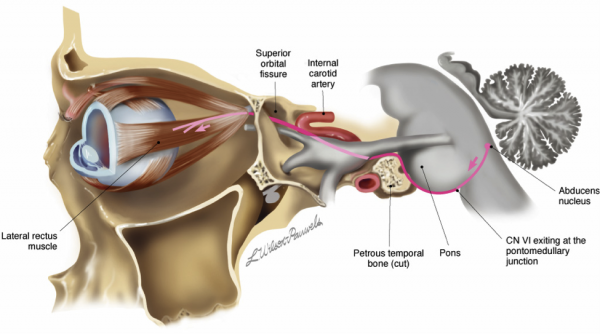
The abducens nerve originates from the sixth nerve nucleus in the dorsal pons, ventral to the fourth ventricle (Figure 1). The fibers exit the nucleus and leave the brainstem at the pontomedullary groove, generally located medial and caudal to facial and vestibulocochlear nerve. The nerve then courses through subarachnoid space to reach the upper edge of the tip of the petrous bone (part of the temporal bone) towards the clivus. This portion of the petrous bone is where the nerve is most susceptible to mastoiditis, otitis or bone infection of any sort. Passing the clivus, the abducens nerve is then surrounded by a fibrous bone-less sheath, known as Dorello's canal, where the nerve is sensitive to stretching with changes in intracranial pressure. After exiting Dorello’s canal, it then enters the cavernous sinus and follows the internal carotid artery before entering the orbit through the superior orbital fissure to innervate the lateral rectus [4].
Figure 1. Diagram of the abducens nerve pathway. Note that the trigeminal nerve (gray nerve exiting the pons) and the abducens nerve (red line) run together adjacent to the petrous bone. Image obtained from AAO.org.
Risk Factors
Patients who have extensively pneumatized petrous apices are more at risk of developing the symptoms of GS. In these patients, ear infections can spread into air spaces within the temporal bone, increasing the risk of compromising nearby nerve structures [5]. Patients with cholesteatomas and chronic osteomyelitis are also at higher risk [6][7]. Diabetics, patients on high dose steroids, and immunosuppressed individuals may be at higher risk of GS because of a weakened ability to limit the spread of infection [8].
General Pathology
GS may progress from untreated otitis media when the infection spreads to the petrous apex of the temporal bone. Infection is commonly caused by Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas, and Staphylococcus aureus [9], while other rare causes include Tuberculosis and fungal infection [10].
Pathophysiology
Bacteria travels from the middle ear to the mastoid air cells, which contain highly vascular marrow and are susceptible to infection [5]. It can then spread to the adjacent petrous temporal bone, around which are located many key structures. These structures include the trigeminal ganglion and abducens nerve,' which are separated from the petrous bone only by dura mater [9]. Inflammation in this region can damage these nerves, causing facial pain and horizontal diplopia secondary to unilateral esotropia.
Primary prevention
The administration of antibiotics upon diagnosis of otitis media helps prevent the progression to GS. [9].
Diagnosis
GS is diagnosed by the clinical triad:
- Otitis media
- Pain in distribution of ophthalmic and maxillary branches of trigeminal nerve
- Abducens nerve palsy
History
Otorrhea and otalgia typically precedes diplopia and facial pain.
The timing of presentation of diplopia and facial pain varies with patients, and due to the rarity of GS it is difficult to determine a typical timeline of presentation for the clinical triad of symptoms. Furthermore, it is difficult to distinguish between facial pain that arises from trigeminal nerve lesion and a radiating headache that develops from a different cause also associated with infection and illness.
In four GS cases, diplopia was observed within a week of otorrhea [1][6][11][9]; in two cases, it was observed within two weeks [12][13]; in another two cases, within a month of otorrhea [14]; in one case, within two months [14]; and in one case, a year after presentation of chronic otorrhea. Notably, the delayed development of diplopia (after at least one month) was found in four of the five cases of prolonged otorrhea (at least one-month history of otorrhea).
There are rare cases where the ear infection is subclinical and where other cranial nerves may be affected, such as CN IX and X[15].
Physical examination
The tympanic membrane should be examined for discharge. Extraocular muscle function, especially the lateral rectus, should also be tested.
Signs
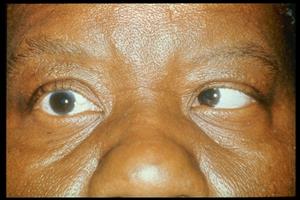
The three classic signs of GS are: otorrhea, facial pain, and horizontal diplopia.
Figure 2: Image showing right sixth nerve palsy. Patient is attempting to gaze to the right. Image obtained from AAO.org.
Symptoms
Patients may complain of earache, tenderness, fever, ear discharge, deep unilateral facial pain, headache, diplopia, dizziness, nausea, vomiting, and confusion [1][13].Rarely, GS can present with a headache and no ear complains, along with other CN involvement (IX,X)[16]
Diagnostic procedures
- CT imaging of the petrous temporal bone is the exam of choice to identify lesions and has a low false-positive rate. It can evaluate the extent of pneumatization of the temporal bone and marrow formation [5].
- MRI imaging of the petrous temporal bone provides more information about the composition of the lesion that CT scans are not able to identify. This is important because it can differentiate between petrous apicitis, osteomyelitis, cholesteatoma, and neoplasms, and therefore guide to appropriate treatment [5][17].
Laboratory test
- Fluid from otorrhea is cultured for antibiotic selection
- Complete blood count
- C-reactive protein
- ESR
Differential diagnosis
- Cholesteatoma, or implantation of epithelium by trauma to the tympanic membrane, can grow to a size that puts necrotic pressure on the petrous bone. [5]
- Petrous effusion is fluid that drains into the air cells of the petrous temporal bone. This may be asymptomatic but can also form cysts. [5]
- Cholesterol granuloma occurs when red blood cells and other tissue break down, releasing cholesterol to form crystals that induce an inflammatory response. These can form granulomas that destroy portions of the temporal bone. [5]
- Osteomyelitis may develop following chronic infection of the external ear, middle ear, mastoid, or petrous apex. [5]
- Neoplasms such as meningioma, intracranial plasmacytoma, chondroma and chondrosarcoma of the petrous temporal bone are very rare [5][18]
Management
Antibiotics are given for an extended period. In more serious cases, other procedures such as myringotomies, placement of ventilation tubes, and surgery may be performed. There is no current guideline for determining when to move from conservative treatment, which risks deterioration of the cranial nerves, to more invasive surgical procedures [11][9].
General treatment
High dose antibiotic therapy is the primary form of treatment. In cases where inflammation puts key structures at risk, surgery is performed, most commonly mastoidectomies and petrosectomies [9].
Medical therapy
High-dose antibiotic treatment is the most common form of medical therapy. Multiple reported cases of GS involved infection by Staphylococcus or Streptococcus bacteria [1][11][19]. Cephalosporins were the most common antibiotics prescribed, in addition to penicillin, ampicillin, and vancomycin. In other cases, metronidazole was prescribed [12].
Other forms of treatment may be used in atypical cases to address the underlying illness. One case of non-Hodgkin’s lymphoma with associated GS was administered chemotherapy, with partial improvement to nerve functions [7].
GS may need to be treated aggressively with antibiotics and the major morbidity is hearing loss.[11] .
Surgery
Patients who fail, are intolerant of, or noncompliant with maximal medical therapy may require mastoidectomy or petrosectomy which removes granulation tissue and necrotic bone that results from infection in either the mastoid cells or the petrous portion of the temporal bone [3][20][21]. A mastoidectomy is performed by making a retroauricular incision to access the mastoid antrum and the mastoid cells deep to it [21]. Using an electric burr and curette, mastoid cells lining the sigmoid sinus and the middle and posterior cranial fossas are removed [21]. A petrosectomy is a similar surgery involving the petrous portion of the temporal bone. It is also performed to remove diseased tissue and reduce further risk of bone erosion and inflammation at the petrous temporal bone [3].
Another related surgical method is an infralabyrinthine bony dissection, in which a burr is used to access the infralabyrinthine air cell tract and then the petrous apex of the temporal bone [11]. Purulent secretions can then be drained and the cavity irrigated with saline and hydrogen peroxide [11]. A tube may be placed to avoid recurrence [11].
Myringotomy, an incision at the tympanic membrane, can be performed to drain fluid. Ear ventilation tubes may also be placed into the ear for the same long-term function [11][3].
Surgical follow up
Otolaryngology should see the patient if surgical treatment is necessary.
Complications
Complications may arise from the spread of infection in several pathways: directly through the temporal and mastoid bone; through the labyrinth via the round window and oval window; or via the bloodstream or thrombophlebitis [22].
Complications include:
- Meningitis [8]
- Intracranial, prevertebral or parapharyngeal abscess [8]
- Spread of infection to cavernous sinus [22]
- Spread to skull base (Vernet's syndrome) [8]
- Hearing loss [11][21]
- Epidural or subdural empyema [22]
- Sigmoid sinus thrombosis [22]
- Hydrocephalus [22]
- Death [13]
Prognosis
GS is rare and becoming even more rare since the advent of better medical and surgical treatment. Documented case reports suggest that in serious cases, treatment with antibiotics and surgery resolves symptoms of otorrhea, horizontal diplopia, and facial pain. However, some patients may not fully recover complete nerve function [8][6][10]
Most cases of GS have full resolution of signs and symptoms after conservative antibiotic treatment, but some cases required surgery [9][19].
If untreated GS can be fatal especially in resource limited nations. One pediatric case from Zimbabwe described a 9-year-old female admitted for discharge in right ear and a year-long history of chronic suppurative otitis media. Upon declination of surgery, the patient developed the clinical triad of Gradenigo’s at day 14, and on day 25, she was declared brain dead [13].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Valles JM, Fekete R. Gradenigo Syndrome: Unusual Consequence of Otitis Media. Case Reports in Neurology. 2014;6:197-201.
- ↑ 2.0 2.1 Hafidh MA, Keogh I, McConn R, Walsh M, Rawluk D. Otogenic intracranial complications. A 7-year retrospective review. American Journal of Otolaryngology. 2006;27(6):390-395.
- ↑ 3.0 3.1 3.2 3.3 Visosky AMB, Isaacson B, Oghalai JS. Circumferential Petrosectomy for Petrous Apicitis and Cranial Base Osteomyelitis. Otology and Neurotology. 2006;27:1003-1013.
- ↑ Nguyen V, Dulebohn SC. Neuroanatomy, Cranial Nerve 6, Abducens (CN VI, Abducent) [Updated 2017 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2017 Jun-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430711
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Jackler RK, Parker DA. Radiographic Differential Diagnosis of Petrous Apex Lesions. The American Journal of Otology. 1992;13(6):562-573.
- ↑ 6.0 6.1 6.2 Taklalsingh N, Falcone F, Velayudhan V. Gradenigo’s Syndrome in a Patient with Chronic Suppurative Otitis Media, Petrous Apicitis, and Meningitis. American Journal of Case Reports. 2017;18:1039-1043.
- ↑ 7.0 7.1 Pedroso JL, de Aquino CCH, Abrahao A, de Oliveira RA, Pinto LF, Bezerra MLE, Silva ABG, de Macedo FDB, de Melo Mendes AV, Barsottini OGP. Gradenigo’s Syndrome: Beyond the Classical Triad of Diplopia, Facial Pain and Otorrhea. Case Reports in Neurology. 2011;3:45-47.
- ↑ 8.0 8.1 8.2 8.3 8.4 Motamed M, Kalan A. Gradenigo’s Syndrome. The Postgrad Medical Journal. 2000;76:559-560.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Burston BJ, Pretorius PM, Ramsden JD. Gradenigo’s syndrome: successful conservative treatment in adult and pediatric patients. The Journal of Laryngology and Otology. 2005;119:325-329.
- ↑ 10.0 10.1 Bhatt YM, Pahade N, Nair B. Aspergillus petrous apicitis associated with cerebral and peritubular abscesses in an immunocompetent man. The Journal of Laryngology and Otology. 2013;127:404-407. Resulting inflammation can damage nearby nerve structures
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 Kantas I, Papadopoulou A, Balatsouras DG, Aspris A, Marangos N. Therapeutic approach to Gradenigo’s syndrome: a case report. Journal of Medical Case Reports. 2010;4:151-154.
- ↑ 12.0 12.1 Jacobsen CL, Bruhn MA, Yavarian Y, Gaihede ML. Mastoiditis and Gradenigo’s Syndrome with anaerobic bacteria. Biomed Central Ear, Nose & Throat Disorders. 2012;12:10.
- ↑ 13.0 13.1 13.2 13.3 Jensen PVF, Avnstorp MB, Dzongodza T, Chidziva C, von Buchwald C. A fatal case of Gradenigo’s syndrome in Zimbabwe and the Danish-Zimbabwean ENT collaboration. International Journal of Pediatric Otorhinolaryngology. 2017;97:181-184.
- ↑ 14.0 14.1 Jensen PVF, Hansen MS, Moller MN, Saunte JP. The Forgotten Syndrome? Four Cases of Gradenigo’s Syndrome and a Review of the Literature. Strabismus. 2016;24:21-27.
- ↑ Bano S, Nawaz A, Asmar A, Aemaz Ur Rehman M, Farooq H, Ali H. Gradenigo's syndrome presenting as IX and X cranial nerve palsy without clinically apparent ear infection: A case report and review of literature. eNeurologicalSci. 2022;27:100397. Published 2022 Mar 17. doi:10.1016/j.ensci.2022.100397
- ↑ Bano S, Nawaz A, Asmar A, Aemaz Ur Rehman M, Farooq H, Ali H. Gradenigo's syndrome presenting as IX and X cranial nerve palsy without clinically apparent ear infection: A case report and review of literature. eNeurologicalSci. 2022;27:100397. Published 2022 Mar 17. doi:10.1016/j.ensci.2022.100397
- ↑ Sattarova, Victoria MD, MSc; Gencturk, Mehmet MD; Lee, Michael S. MD; McClelland, Collin M. MD. Gadoxetate Disodium-Enhanced Imaging of Gradenigo Syndrome in End-Stage Renal Disease. Journal of Neuro-Ophthalmology 41(3):p e375-e377, September 2021. | DOI: 10.1097/WNO.0000000000001218
- ↑ Bourne RRA, MacLaren RE. Intracranial plasmacytoma masquerading as Gradenigo’s syndrome. British Journal of Ophthalmology. 1998;82(4):458-459.
- ↑ 19.0 19.1 Janjua N, Bajalan M, Potter S, Whitney A, Sipaul F. Multidisciplinary care of a paediatric patient with Gradenigo’s syndrome. BMJ Case Reports. Published online 2016.
- ↑ Profant HJ. Gradenigo’s Syndrome with a consideration of “petrositis.” Archives of Otolaryngology. 1931;13(3):347-378.
- ↑ 21.0 21.1 21.2 21.3 Hildmann H, Sudhoff H, Dazert S, Hagen R. Manual of temporal bone exercises. Springer. 2011:5-29.
- ↑ 22.0 22.1 22.2 22.3 22.4 Colpaert C, Rompaey VV, Vanderveken O, Venstermans C, Boudewyns A, Menovsky T, de Veuster I, Van de Heyning P, Hamans E. Intracranial complications of acute otitis media and Gradenigo’s Syndrome. B-ENT 2013;9:151-156.