Glaucoma in the Developing World
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Glaucoma is the most common cause of irreversible vision loss and the third most common cause of vision loss worldwide after cataracts and refractive error.[1] [2] In developing countries, those affected with the disease are at a particular disadvantage in comparison to persons with glaucoma in developed nations; they have a higher incidence of disease, more advanced disease at presentation, and a higher risk of progressing to blindness.[3] [4][5] Glaucoma prevention and treatment has been a major focus of international directives, including the World Health Organization’s Vision 2020 campaign. While this initiative saw a successful decrease in worldwide blindness, most of the improvement was due to widespread adoption of cataract surgery and spectacles provision. Stated goals regarding number of ophthalmologists and optometrists in developing nations remain unfulfilled.[6] [7] [8]
There are several unique challenges to addressing glaucoma in the developing world. Glaucoma is difficult to diagnose in any context; it is almost always asymptomatic until late in the disease course. This painless, insidious vision loss results in late diagnosis unless patients are screened for glaucoma early on. Access to eye care in developing countries remains a major issue, particularly in rural areas. Most ophthalmologists are concentrated in urban areas, leaving many people in resource-limited countries unable to obtain the regular eye exams and glaucoma treatment necessary to prevent permanent vision loss. Understanding or even awareness of glaucoma is virtually nonexistent in some populations, leading to unrealistic expectations of vision restoration through surgery.[9] While this type of surgical outcome is attainable with reversible causes of vision loss such as cataracts, it is impossible for patients with advanced glaucoma to regain functional vision. This misunderstanding is prevalent across the developing world and is only one symptom of a widespread lack of education regarding eye health.[10] [11] Misinformation regarding eye diseases and treatment options also discourages many from seeking any eye care at all.[12]
In resource-limited countries, glaucoma care providers tend to favor a more aggressive treatment approach than that of developed countries. Reliable follow-up is less likely and patients are often unable to obtain or afford medications. Consequently, incisional glaucoma surgery typically is first-line treatment, instead of being reserved for those who fail medical management.[13] [14]
The majority of glaucoma data are collected from European and North American studies. Information from the developing world is limited, but has increased in recent years. This article examines the state of glaucoma in the developing world, with particular attention given to epidemiology, approach to treatment, and outlook for the future.
Epidemiology
The prevalence of glaucoma in developing countries is difficult to pinpoint because of differences in defining glaucoma, variable expertise of diagnosticians, and frequent unavailability of necessary diagnostic equipment. Much of the available epidemiology likely underestimates the true glaucoma burden in developing countries due to reliance on visual acuity thresholds for diagnosing glaucoma.[15] The estimated number of people with glaucoma worldwide is expected to rise from 76 million in 2020 to 111 million in 2040, with Africa and Asia being affected more heavily than the rest of the world.[2][16] The estimated number of glaucoma worldwide is 76 million in 2020, with 8 million suffer from moderate and severe visual impairment (MSVI) and blindness. The total number is expected to increase to 111 million in 2040, with Africa and Asia being affected more heavily than the rest of the world. .[2][16] The total amount of glaucoma care has increased worldwide in recent years, but rapid growth in the global population coupled with demographic shifts toward older populations has limited the impact of this expansion of care on a global per capita basis.[1]
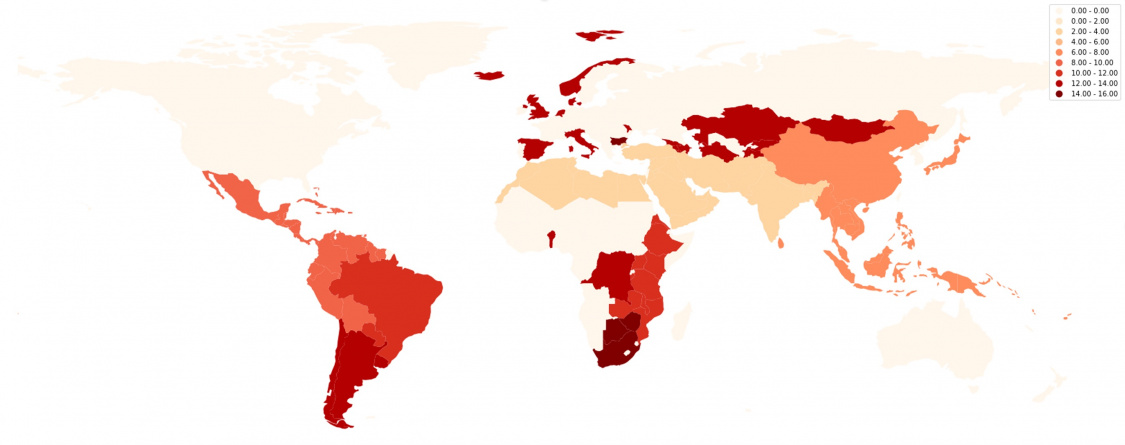
Figure 1: Prevalence of MSVI and blindness due to glaucoma[5] [17] [18] [19] [20] [21]
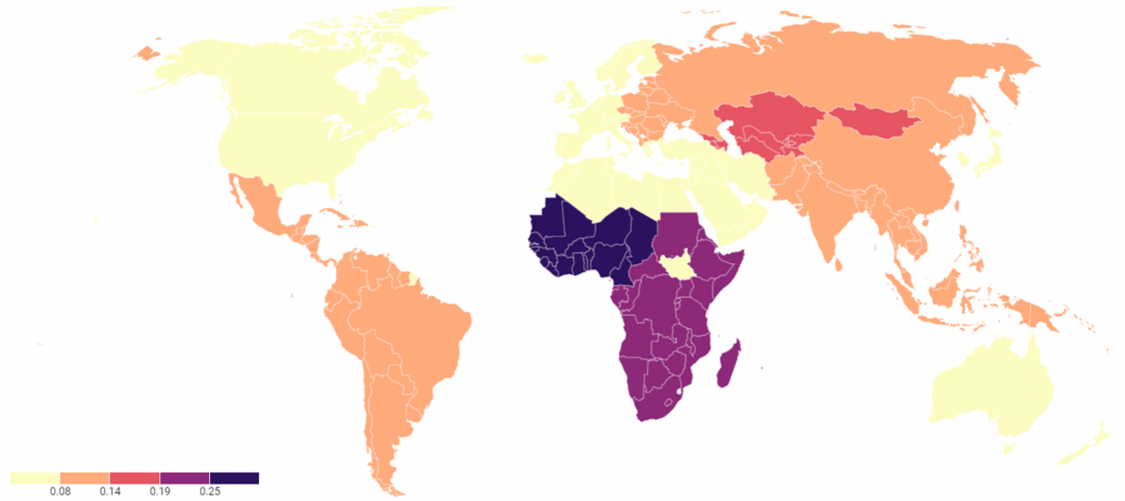
Figure 2: Glaucoma blindness as a percentage of all causes of blindness in each country[5] [17] [18] [19] [20] [21]
Primary open-angle glaucoma
Primary open-angle glaucoma (POAG) is the most common form of the disease worldwide. The highest prevalence of POAG is in African countries and in communities of African descent, such as African American and Afro-Caribbean populations.[16][28] [29] A 2014 review of worldwide POAG prevalence among people aged 40-80 years showed estimates of 2.31% in Asia, 3.65% in Latin America and the Caribbean, and 4.20% in Africa.[16] Another study based in West Africa showed a POAG prevalence of nearly 15% in individuals over the age of 80.[28] Among Asian populations, the prevalence of POAG ranges from 0.5% in a Mongolian population to 3.9% in a Japanese population.[30] [31] [32]
Although several genetic loci have been identifed as “glaucoma genes”, inheritance of POAG is usually multigenic and multifactorial, having no identified association with a particular gene in most populations. Environmental factors and modifying genes likely play a role in disease development.[33] [34] [35]
Primary angle-closure glaucoma
Angle-closure glaucoma is the second most common form of the disease. Primary angle-closure glaucoma (PACG) is responsible for nearly half the glaucoma-related blindness in the world, despite being much less common than POAG. Asia bears the greatest burden of PACG with reported prevalence reaching as high as 2.5% in Myanmar.[30] [36]Females are more likely to have PACG, with a 1.5:1 female to male ratio observed in Asians.[37] Angle closure disease is much less common outside of Asia.
Family history has been identified as a major risk factor for development of angle-closure glaucoma. In an Iranian study, 58% of siblings of patients with angle closure glaucoma were found to have some degree of angle closure.[38] Findings from this study are consistent with previous PACG studies from India and Singapore, in which 50% and 59% of siblings were affected, respectively.[39] [40] [41] It has long been established that angle closure is more common in Asian populations. However, there are only a few studies differentiating between ethnic groups living in a single area.[37][42] [43] A study evaluating the course of angle closure glaucoma in different ethnic groups living in Malaysia found that Malays presented to clinic with higher intraocular pressures (IOPs), worse vision, more advanced optic disc changes, and at an older age than Chinese living in Malaysia. The Malay group also experienced faster glaucoma progression than the Chinese group.[44] [45]
Childhood glaucomas
Childhood blindness is a much larger problem in the developing world than in the developed world. About three-quarters of the world’s blind children live in impoverished areas of Africa and Asia alone.[46] Poorer countries are also more likely to have a larger percentage of blindness in children due to avoidable causes.[46] In a study conducted in Ghana, Honduras, and India, 95% of caregivers believed that it was important for children to have eye exams, but 66% had never undergone one.[47] Accordingly, the prevention and treatment of diseases that cause blindness during childhood are of particular concern to the WHO. The concept of blind years also highlights the importance of addressing childhood blindness; despite its relative rarity, the total number of blind years due to childhood blindness is almost equal to the number of blind years due to adult cataracts.[46]
The most common type of infantile glaucoma in the world is primary congenital glaucoma (PCG), although it is still rare with an incidence of about 1 in 10,000 to 18,000 live births. Significantly higher PCG prevalence has been reported in populations where consanguineous relationships are common.[48] [49] [50] Late diagnosis and advanced disease at presentation are common. One study from Ethiopia found the mean age at diagnosis of PCG to be 3.3 years.[51] This is in contrast to the developed world, where the diagnosis is almost always made within the frst year of life.[49][52] In this Ethiopian case series, the authors suspected a poor long-term prognosis for their patients despite appropriate surgical intervention because of inadequate follow-up and unavailability of medications.
Post-cataract surgery glaucoma is another type of childhood glaucoma that is particularly important in developing nations. Due to the relatively large number of cataract operations performed in a medical mission setting, long-term follow-up to assess for complications is often not feasible. As pediatric cataract surgery rates increase, there is an increasing incidence of related secondary glaucoma. A study conducted at a Tanzanian tertiary pediatric eye center found a 6.5% risk for glaucoma at three years post cataract extraction in children under age 18.[53] Previous studies have reported significantly higher rates of glaucoma development when children are followed for longer time periods after surgery.[47][54] [55] [56] [57]
Secondary glaucomas
Lens-induced glaucoma (LIG) is the most common form of secondary glaucoma in many resource-limited countries. For example, LIG accounted for 5.3% of all causes of glaucoma presenting to a tertiary eye hospital in Nepal between 2007 and 2008.[58] This relatively high prevalence is a refection of the large backlog of advanced cataracts in the developing world.
Other secondary forms of glaucoma such as neovascular and pseudoexfoliative glaucoma have not been studied widely in developing countries. A Nigerian population-based study identified a 0.9% prevalence of all forms of secondary glaucoma. Of those patients diagnosed with neovascular glaucoma, 84% of affected eyes were blind on presentation. The study also identified a 0.2% prevalence of non-neovascular open-angle glaucoma secondary to couching, which is the same prevalence of neovascular cases in the study population. This finding reflects the reality that cataract surgery by couching is still a surprisingly common practice in some developing countries, despite a similar cost and vastly inferior outcomes compared to extracapsular cataract extraction.[59] [60] [61] [62] [63] Diabetes mellitus, which is a major cause of neovascular glaucoma in the developed world, was found in only 8.3% of subjects, while 62% had systemic hypertension.[64] [65] [66] A study on neovascular glaucoma conducted in Saudi Arabia found the most common cause of the disease to be diabetic retinopathy, followed by retinal vein obstruction. A history of diabetes was reported in 65% of subjects. Less common causes included chronic retinal detachment, carotid artery stenosis, retinoblastoma, and uveitis.[67]
In a tertiary eye care center in India, common causes of secondary glaucoma included vitrectomy surgery (14%), trauma (13%), corneal pathology (12%), aphakia (11%), neovascular glaucoma (10%), pseudophakia (10%), steroid-induced glaucoma (8%), uveitic glaucoma (8%), and miscellaneous causes (14%).
When grouped by patient age, the most common type of secondary glaucoma for 0-20 years of age was trauma; post-vitreoretinal surgery for 21-40 years of age; neovascular glaucoma for 41-60 years of age; and pseudophakic glaucoma for those over 60 years of age.[68]
Pseudoexfoliative glaucoma is a relatively uncommon form of secondary glaucoma in most regions of the developing world. However, one clinic in Ethiopia has reported it as the most common subtype of glaucoma, identified in 35% of glaucoma patients.[69] In a South Indian population study, pseudoexfoliation was identified in 3.7% of subjects, and of these 8.3% had associated glaucoma.[70]
Psychosocial and economic impact
Poor health is strongly associated with low socioeconomic status in the developing world, and eye disease is no exception.[71] [72] Glaucoma specifically can have a potentially devastating impact on quality of life, and glaucoma’s irreversible of vision loss makes early diagnosis and treatment all the more critical. On a quality of life scale from 0 to 1, where 0 represents death and 1 represents perfect health, an Indian population with glaucoma reported a mean utility value of 0.64, much lower than the score reported a similar study in U.S. citizens with glaucoma. Brazilians with glaucoma also report a range of utility values lower than their U.S. counterparts. This suggests persons with glaucoma in developing countries feel a stronger impact on their quality of life.[73] [74] Not surprisingly, the degree of impact on quality of life is closely correlated with degree of visual impairment across all areas of the developing world and severity of low vision is significant predictor of quality of life.[75] [76] [77][17]
An often-overlooked effect of blindness is the secondary impact on those around a given blind person. Caregivers of vision impaired persons often bear a significant burden related to financial stress, low education, and being forced to become a caregiver.[78] A study in India reported an increased prevalence of depression in caregivers of persons with low vision or blindness. The rate of caregiver depression increased from 16% in the 20/200 group to 48% in the no light perception group. Low income was also associated with higher rates of depression.[79]
Blindness often removes two people from the workforce: the blind patient, and a family member to care for the patient.[71] Thus, glaucoma’s impact spreads far beyond individuals and their families; vision impairment and blindness have a major impact on local and national economies.[80] [81]
Glaucoma management and its challenges
Diagnosis
Universal community screening for glaucoma in developing countries is not economically feasible due to the limited numbers of providers capable of performing screenings, as well as financial and transportation barriers.[82] [83] [84] Many patients initially present when they become symptomatic from glaucoma or another ophthalmic issue. Consequently, late diagnosis is much more common in resource-limited countries.[85] For example, a study conducted in a rural population in northeastern Ghana showed bilateral blindness in 34% and unilateral blindness in half of patients receiving an initial glaucoma diagnosis.[86] This is in line with several other studies in Sub-Saharan Africa, which report a unilateral blindness rate of up to 56% in newly diagnosed glaucoma patients.[87]
Due to limited diagnostic resources, diagnosis is often made based on IOP, vision, and examination without any ancillary testing such as formal visual fields and OCTs. One recent survey of Nigerian glaucoma management found that basic diagnostic equipment was unavailable in 15-20% of clinics.[88] Gonioscopy is not always feasible at the point of screening due to a lack of access to the necessary equipment and/or personnel trained at performing gonioscopy. Thus, the use of van Herick grading or even oblique flashlight grading is more often utilized in screening for occludable angles.[84]
Medical management
Medical management of glaucoma in developing countries is more difficult than in developed countries due to more advanced disease at time of presentation, limited access to ophthalmic medications, lack of affordability of medications, variable quality of generic ophthalmics, and poor follow-up.[89] [90] [91] A study from India highlighted the significant economic burden caused by anti-glaucoma drug regimens, particularly on poorer people from rural areas. The monthly cost of glaucoma medications represented 13%-123% of monthly income for the patients in the lowest socioeconomic groups. This did not take into account the lost income or costs of travel for their appointments, which are often greater than the cost of the medicine itself. The vast majority did not have any type of insurance or reimbursement for costs of treatment.[92] As such, glaucoma medical management in the developing world typically is reserved for patients with early glaucoma whose medication adherence and follow-up are all but certain or for patients who decline surgery. It was reported that medication compliance ranged from 32.5% to 65.4% in Sub Saharan Africa countries.[18]
Surgery
Surgery is generally regarded as the preferred first-line treatment for glaucoma in developing countries.[93] [94] [95] This is due to late diagnosis, poor follow-up, and the aforementioned difficulties of medical management. Small prospective trials of early glaucoma surgery in developing countries have demonstrated favorable outcomes and patient satisfaction.[96] [97] Despite this, patient acceptance of surgery in developing countries is highly variable and often low.[87][96][97] Patient-related barriers to surgery include unfamiliarity with glaucoma, absence of symptoms at time of diagnosis, and fear of complications. Due to a lack of knowledge about the disease, many newly diagnosed patients with end-stage glaucoma in their worse eye decline potentially vision-saving surgery on their better eye.
One survey of Nigerian ophthalmologists identified multiple surgeon-specific concerns that may act as barriers to surgical intervention as well, including concern about lack of patient satisfaction, complications of surgery, and negative publicity.[89] Such concerns are not unfounded. Glaucoma surgery is designed to prevent vision loss, but patients with glaucoma who lack eye health literacy often have an expectation for sight restoration, such as through spectacles or cataract surgery. In practice, performing incisional glaucoma surgery on patients in a population where there is little understanding of eye diseases can potentially discourage patients with cataract blindness and other reversible causes of vision loss from seeking eye care. Nevertheless, glaucoma surgery acceptance rates can substantially increase when patients are properly educated about the disease at the time of diagnosis.[96]
Trabeculectomy is the most common glaucoma surgery performed in the developing world.[94][98] [99] [100] When performed with antimetabolites (e.g. mitomycin C or 5-fuorouracil), the surgery has a reasonably high rate of providing significant long-term IOP reduction.[101] [102] Trabeculectomy is arguably the most cost-effective glaucoma intervention because it requires minimal equipment, does not involve the implantation of a device that may be prohibitively expensive or difficult to obtain, and often obviates the need for long-term medical management.[95] The surgery is highly effective for not only open-angle glaucoma but also for angle-closure forms of the disease; in countries with a high incidence of angle-closure glaucoma (ACG) such as China, trabeculectomy is the preferred first-line treatment for PACG with significant peripheral anterior synechiae.[14]
Modifications to what many surgeons in developed countries would consider a “standard” trabeculectomy technique are common in resource-limited parts of the world. Surgeons often employ releasable sutures due to the unavailability of lasers for suture lysis.[97] Releasable sutures also enable a tighter suturing of the scleral fap, which reduces the incidence of postoperative hypotony and its sequelae, including the acceleration of cataract formation.[103] Even with optimal technique, trabeculectomy in any form is cataractogenic, especially when antimetabolites are used. Patients in the developing world also frequently present with both visually significant cataracts and glaucoma. To address both issues, techniques have been developed that combine manual small incision cataract surgery (MSICS) with trabeculectomy.[104] Some have advocated for the use of beta radiation as an alternative to antimetabolites in developing countries because a radiation delivery device is reusable, durable, and does not depend on regular supply of medication.[85][99]
There is an increased risk of trabeculectomy failure in African eyes compared to Caucasian eyes.[105] [106] While the use of antimetabolites significantly reduce the risk of failure, this racial difference may not be present with tube-shunt surgery.[107] [108] A small group of adult and pediatric cases receiving the Ahmed valve implant in Kenya showed IOP reduction from a mean of 36.4 mmHg to 16.7 mmHg along with a decrease in medication requirement and only one major complication.[109] In Ethiopia, a series of patients with refractory glaucoma received tube-shunt placement surgery and experienced similar success.[110] Given the decreased follow-up burden with tube placement as compared to trabeculectomy, decreased need for reoperation, and comparable number of medications needed to maintain goal IOP; tube placement may be a viable choice for disease management in developing countries.[102] [111]Potential barriers to tube-shunt placement in the developing world are added cost, access to the device, and access to the donor tissues typically used to cover the tube at its entry site (e.g. cornea, sclera and pericardium). Aurolab, the nonprofit manufacturing unit of the Aravind Eye Care System, manufactures the Aravind Aqueous Drainage Device, a valveless tube-shunt that is comparable to the Baerveldt 350 glaucoma implant. This device is sold throughout the world for approximately US$50 - a fraction of the cost of other glaucoma tube-shunts.
Recent research on the use of intracameral moxifoxacin as the last step of cataract and glaucoma surgery in the developing world has found a robust decrease in the incidence of postoperative endophthalmitis.[112] One retrospective study reported a nearly 4-fold reduction in early endophthalmitis following trabeculectomy or combined trabeculectomy/cataract extraction surgery.[113] In response, many surgeons and eye care systems throughout the world have adopted intracameral moxifoxacin as standard practice for glaucoma surgery.
For ACG, cataract extraction with or without trabeculectomy may be a reasonable surgical option. The crystalline lens plays a major mechanistic role in ACG by virtue of its size, in effect mechanically crowding anatomically narrow-angled eyes. Additionally, because trabeculectomy accelerates the development of cataract, combining the two surgeries or simply performing standalone cataract extraction may provide better long-term visual outcomes in developing regions where patient follow-up is poor. One randomized controlled trial of eyes with medically controlled primary angle-closure glaucoma (PACG) and cataract, demonstrated that phacoemulsifcation alone garnered IOP reductions similar to combined phacoemulsifcation/trabeculectomy.[114] In a related study of eyes with medically uncontrolled PACG, the combined surgery group had greater IOP reductions than the standalone phacoemulsifcation group. Notably, phaco-trabeculectomy also yielded more complications.[115]
Clear lens extraction for the treatment of angle-closure glaucoma is controversial. In younger patients, clear lens extraction results in the loss of accommodation and also confers a higher risk of rhegmatogenous retinal detachment.[116] Nevertheless, one prominent multicenter randomized controlled trial found that for the management of PACG and primary angle closure with elevated IOP, clear-lens extraction had superior outcomes and was more cost-effective than laser peripheral iridotomy.[117] Another randomized controlled trial investigating management of uncontrolled PACG in eyes without cataract found that trabeculectomy was superior to phacoemulsifcation in terms of IOP and medication reduction.[118] However, trabeculectomy also was associated with more complications in the study.
Management of neovascular glaucoma (NVG) is notoriously difficult in any setting. In general, glaucoma drainage implants are considered more effective than trabeculectomy in treating NVG because fibrovascular membranes can grow over and obstruct trabeculectomy sclerostomy sites. However, trabeculectomy can successfully treat NVG in the developing world, especially when antifibrotics and anti-VEGF agents are also used.[119] Multiple challenges limit the use of bevacizumab and other anti-VEGF agents in the developing world, including cost, availability, and the need for refrigerated storage.
Lasers
Selective laser trabeculoplasty (SLT) is popular in settings where the necessary equipment is available. In a population of African descent in St. Lucia, SLT treatment of patients with open-angle glaucoma had a success rate of 77.7%, with success defined as at least 10% reduction in IOP. About half of the successful eyes had at least a 40% reduction in IOP from post-washout baseline.[120] A Chinese study of SLT reported a success rate of 53%, using an IOP reduction of at least 20% as the cutoff for success.[121] In Africa and other developing regions of the world, SLT may be a better alternative to surgery than medical therapy because of the considerable challenges with medication compliance in resource-limited countries.[122]
Laser peripheral iridotomy (LPI), where available, is a reasonable and relatively safe alternative to surgery for the management of primary angle-closure or “early” PACG. However, there is growing evidence that PACG with definite glaucomatous optic neuropathy does not respond well to LPI. One recent review of the literature found that in 68% to 94% of PACG patients initially treated with LPI, the IOP actually increased at 6 months post-laser.[14] Reflecting this reality, Chinese medical textbooks generally recommend trabeculectomy as the first-line treatment for PACG with peripheral anterior synechiae > 180 degrees.
Cyclodestructive procedures generally are viewed as last-line treatment for patients with poor vision due to their side-effect profle (i.e. uveitis, cataract, ischemia, hypotony, phthisis), but there has been some support for using cyclophotocoagulation as frst-line treatment in developing countries.[123] In Malawi,47 eyes with primary open-angle or pseudoexfoliative glaucoma were treated with low-dose transscleral diode laser cyclophotocoagulation. Half of these patients maintained an IOP reduction of at least 25% at 3 months.[124] Similar results were reported in Tanzania and Cameroon.[125] [126] The development of micropulse transscleral diode lasers such as the MicroPulse P3 (Iridex, Mountain View, CA) offers an additional alternative to medication and incisional surgery.[127] Because micropulse lasers are less destructive than traditional continuous wave diode lasers, they have a better safety profile, but the IOP-lowering effects are not as robust and may not be as sustained.
Potential solutions and future directives
Diagnosis
Despite recent efforts such as the VISION 2020 global initiative to eliminate avoidable blindness, most people with glaucoma remain undiagnosed.[15] Because population-based screening is resource-prohibitive, the WHO and many global ophthalmology experts advocate for enhanced opportunistic screening.[83] For example, a cost-effective strategy at improving glaucoma diagnosis rates is the promotion of comprehensive eye examinations for people all above 40 years of age.[84] The development of novel technologies and the repurposing of existing technologies offer considerable promise for improving glaucoma diagnostics in the developing world.
Teleglaucoma
The SARS-CoV-2/COVID-19 pandemic has had a profound impact on global healthcare, and ophthalmology is one of the most impacted medical specialties.[128] [129] With social distancing measures in place, ophthalmology practices in both developing and developed countries have rapidly adopted telemedicine out of necessity.[130] [131] [132] [133] [134] [135] [136]
Notable advantages of teleophthalmology, not necessarily limited to times of crisis, include decentralization of care, cost-efficiency, time-efficiency and high patient satisfaction.[130] [131] [132] [133] [134][137] [138] [139] [140] Potential advantages unique to resource-limited and remote areas worldwide include expanded access to expert eye care, facilitation of medical management as an alternative to surgery, and improved disease outcomes.[141] [142] [143] [144] [145] [146] These benefits are particularly relevant for the subspecialty of glaucoma due to its heavy reliance on testing to guide management decisions.[147]
Teleophthalmology has been implemented in some developing countries with success, albeit on a limited scale. This important technology is convenient and can potentially decrease the cost and reduce the number of patients with advanced glaucoma. [19][20] Two commonly employed telemedicine models are “real-time” and “store-and-forward.” In a “real-time” model, healthcare professionals and patients directly interact via audio or audiovisual communications. This interaction may include live collection of objective data such as visual acuity. In a “store-and-forward” model, patients receive a diagnostic workup at a remote ophthalmic testing center or primary health care center equipped with the necessary diagnostic equipment. This workup is then sent electronically to healthcare professionals elsewhere, such as in major eye centers, where the data is interpreted at a later time.[148] [149] [150] [151] For example, a store-and-forward teleglaucoma program utilizing Van Herick angle grading in combination with pachymetry has been shown to have an acceptable sensitivity and specificity for diagnosing occludable angles.[152] Store-and-forward teleophthalmology has been successfully implemented in long-term, remote care of glaucoma patients.[153] Real-time video visits with data from remote testing centers can help guide management without the need to bring the patient in office, unless there are signs of worsening.[147][154]
Widespread implementation teleophthalmology remains a considerable challenge in resource-limited regions of the world, in part due to inadequate telecommunications infrastructure and poor integration of ophthalmology with existing healthcare systems.[141] [142] [143] [144] [145] [146][21]
Smartphone-based testing
Smartphone-based glaucoma testing is a promising area of diagnostics. Such technology’s portability, relatively low cost, and potential use without the physical presence of an ophthalmic specialist make smartphone-based testing attractive for serving rural and under-resourced populations. Furthermore, the increasing ubiquity and sophistication of smartphones potentially enables widespread adoption of such technologies in developing countries.[137] [138] [139] [140]
The use of smartphone photography for ocular imaging has been validated as safe.[155] Smartphone photography for anterior segment screening examination,[156] [157] gonio-imaging,[158] and fundus and disc assessment[137][159] [160] [161] [162] [163] [164] [165] has been described in the literature. Smartphone and tablet-based visual field applications such as Melbourne Rapid Fields (MRF) have been developed and offer screening comparable to tangent screen testing.[166] Virtual reality based visual field testing also may be a portable alternative to office based visual field testing, with the added benefits of gaze tracking capabilities.[167] Proof-of-concept smartphone systems have even demonstrated the potential to estimate IOP with reasonable accuracy.[168]
Artificial intelligence (AI) and deep learning
New avenues with automated glaucoma screening and diagnostics are being explored using machine learning and AI. In settings where specialist care is scant, artificial intelligence may provide potentially powerful screening and diagnostic tools that can be integrated with existing primary care delivery models in the developing world.[169] [170] [171] The division of machine learning that focuses on imaging is deep learning. Possible barriers to widespread application include cost of development and implementation as well as a lack of an ethnically diverse data set.[172]
Automated Screening and Diagnostics
AI-based systems have demonstrated remarkable capabilities in analyzing retinal images, optic nerve scans, and visual field tests to detect signs of glaucoma with high accuracy.[173] By employing machine learning algorithms, these systems can identify subtle changes indicative of glaucomatous damage at early stages, enabling timely intervention and management.[174][175] Moreover, AI-powered tools can streamline the screening process, reducing the burden on healthcare providers and improving patient access to essential eye care services.[173]
Integration with Primary Care Delivery Models
One of the key advantages of AI-driven glaucoma screening is its potential for integration with existing primary care delivery models prevalent in the developing world.[176] By deploying AI-enabled devices in community health centers, mobile clinics, and telemedicine platforms, primary care providers can enhance their capacity to detect and manage glaucoma cases at the grassroots level. This decentralized approach ensures broader coverage and facilitates proactive management of ocular health in underserved populations.
Deep Learning and Imaging Analysis
Deep learning, a subset of machine learning, has emerged as a powerful tool for image analysis in glaucoma diagnosis.[177] By training neural networks on large datasets of retinal scans, optical coherence tomography (OCT) images, and visual field examinations, deep learning algorithms can discern intricate patterns and subtle abnormalities indicative of glaucomatous damage. This advanced level of analysis complements traditional diagnostic methods and enhances the accuracy of glaucoma detection, especially in cases where expertise is limited.[178]
Challenges and Considerations
Despite the potential benefits of AI and deep learning in glaucoma detection, several challenges hinder their widespread implementation in the developing world. The cost of developing and deploying AI-based solutions, including hardware, software, and training, poses a significant barrier, particularly in resource-constrained settings.[179][180] Moreover, the lack of ethnically diverse datasets for algorithm training raises concerns about the generalizability and accuracy of AI models across different populations.[181] Addressing these challenges requires collaborative efforts among researchers, healthcare providers, policymakers, and technology developers to develop cost-effective and culturally sensitive AI solutions tailored to the needs of diverse communities.
Patient education and awareness
One of the largest barriers to eye care in developing countries is the lack of community awareness about common eye diseases and their treatment. Currently there is widespread lack of understanding regarding glaucoma’s causes, symptoms, and natural history. In a multicenter study from Nigeria, only 46% of patients were aware that glaucoma causes vision loss and 73% believed that vision loss due to glaucoma was reversible.[98] In Botswana, 11.5% of patients with previously diagnosed glaucoma had ever heard of the disease before the diagnosis and 36% still did not understand the disease after being diagnosed.[9] In India, awareness, or being familiar with the term glaucoma, was found to be 2.3% in an urban population and 0.32% in a rural population.[182] [183] All of these numbers are much lower than the awareness and understanding of disease reported from developed countries.[184] [185] Lack of patient knowledge about glaucoma has been linked to refusal of surgery, poor medication adherence, more psychological depression, and a lower quality of life.[186] [187] [188] To address this knowledge gap, glaucoma experts have called for the development of culture-specific marketing and other local glaucoma public service awareness programs that increase general knowledge about glaucoma and promote routine vision screening.[84]
Medical management
As in developed countries, medication adherence in the developing world is a major obstacle to nonsurgical management of glaucoma. The recent development of sustained-release drug delivery systems such as bimatoprost SR (Allergan, Dublin, Ireland) portends considerable promise, although the current cost and availability of such medications all but preclude their use in resource-limited settings.[189] Lowering the cost and improving the quality of generic ophthalmic medications throughout the developing world is critical to more widespread medical management of glaucoma. Earlier diagnosis and improved monitoring such as via emerging teleglaucoma technologies may increase the role of medications worldwide.
Eye care professional education
The expansion of professional eye care education is critical to adequately addressing glaucoma in the developing world. While most countries have increased their total number of eye care professionals in recent years, the number of ophthalmologists, optometrists, and other eye care professionals remains inadequate.[83] In particular, increasing the numbers of ophthalmic paraprofessionals in resource-limited countries is essential to providing the routine vision screenings necessary to detect and treat glaucoma. Improved distribution of eye care professionals is also needed to address the world’s uneven distribution of glaucoma and other eye diseases. One global survey found that two thirds of the world’s ophthalmologists reside in just 13 countries.[190]
To improve quality of ophthalmic training in developing countries, which can be variable, some countries such as several in Eastern, Central, and Southern Africa have made recent efforts at standardizing training curricula.[191] The expanded use of structured wet lab courses and specialized artificial eyes also may play a key role in the improved quality and volume of eye surgeon training in areas of greatest need.[192] Some ophthalmology institutions including Aravind Eye Hospitals in India, Tilganga Institute of Ophthalmology in Nepal, LV Prasad Eye Institute in India, Byers Eye Institute at Stanford University, and Moran Eye Center at the University of Utah have created global ophthalmology fellowships and international observership programs as a means of improving ophthalmology training in developing countries. Orbis International, a nonproft organization based out of the United States, fosters skills transfer to eyecare professionals in developing countries via its “Flying Eye Hospital” MD-10 aircraft and via Cybersight, Orbis’s teleophthalmology and online education platform.[193]
Eye care integration into existing systems
Since 1984, the WHO has recommended integrating eye care into existing healthcare systems.[194] Specifically, many experts advocate for expanding the role that primary care systems play in detecting common eye diseases such as glaucoma.[15] Such an approach is not necessarily straightforward, and unfortunately in places like Africa, little ground has been gained since 1984 toward these ends.[194] Most primary health workers worldwide have poor knowledge of glaucoma. Existing healthcare systems are often resource-limited as it is, so the added burden of screening for eye diseases may not be feasible without additional financial and material resources. Even when primary care physicians in developing countries identify vision issues, referral pathways to an eye care specialist are often lacking.[90]
Successful, self-sustaining models for comprehensive eye care in the developing world do exist, such as LV Prasad Eye Institute, Tilganga Institute of Ophthalmology, and Aravind Eye Hospitals.[90][195] These eye care systems are multi-tiered and have excellent referral pathways, from village screenings to dedicated eye hospitals. Eye care for the poor is cross-subsidized by eye care for those who can pay and elect for premium services. Organizing glaucoma care into multiple levels of care such that screening is performed at a primary level, medical care is performed at a secondary level, and surgery is performed a tertiary level, enables a broader reach of eye care into rural and resource-limited regions.[84] The Indian government’s m-Health mobile telemedicine system may constitute a viable model for glaucoma management in developing countries, especially as internet access becomes more ubiquitous.[196] Artificial intelligence- and machine learning algorithm-assisted interpretation of ophthalmic imaging may play a critical role in making glaucoma screening affordable and accessible in remote parts of the world.[172]
Conclusion
Glaucoma continues to pose a major challenge in developing countries. Unlike blindness related to vitamin A deficiency, onchocerciasis, and other vision-impairing diseases that have been addressed through public health initiatives, glaucoma management requires one-on-one care from an eye care specialist. Unlike cataract blindness, which can be reversed permanently with a single surgery, glaucoma care generally necessitates routine follow-up for monitoring of disease progression, medication adjustments, procedures, and/or surgeries. Emphasis should be placed on training, educating, and mobilizing existing non-ophthalmology health care systems. Improved training and retention of eye care specialists in developing countries is critical to expanding global access to glaucoma care. Further development of teleglaucoma and its successful integration into existing models of healthcare delivery may dramatically improve access to eye care in resource-limited countries. Continued efforts through multinational initiatives, public health education, professional training programs, and programs focused on early disease detection and treatment are all needed for vision loss to be decreased in the developing world. Since glaucoma is more prevalent in many developing countries than in the developed world, it should be a core focus of any campaign aimed at improving eye health.
Additional Resources
- International Association for the Prevention of Blindness. Vision Atlas. https://www.iapb.org/learn/vision-atlas. Accessed November 11, 2020.
References
- ↑ Jump up to: 1.0 1.1 Flaxman SR, Bourne RR, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. The Lancet Global Health 2017; 5(12): e1221-e34.
- ↑ Jump up to: 2.0 2.1 2.2 Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121(11): 2081-90.
- ↑ Chen PP. Risk and risk factors for blindness from glaucoma. Current opinion in ophthalmology 2004; 15(2): 107-11.
- ↑ Omoti A, Osahon A, Waziri-Erameh M. Pattern of presentation of primary open-angle glaucoma in Benin City, Nigeria. Tropical doctor 2006; 36(2): 97-100.
- ↑ Jump up to: 5.0 5.1 5.2 Bourne RR, Jonas JB, Bron AM, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2018; 102(5): 575-85.
- ↑ Ackland P. The accomplishments of the global initiative VISION 2020: The Right to Sight and the focus for the next 8 years of the campaign. Indian journal of ophthalmology 2012; 60(5): 380.
- ↑ Palmer JJ, Chinanayi F, Gilbert A, et al. Trends and implications for achieving VISION 2020 human resources for eye health targets in 16 countries of sub-Saharan Africa by the year 2020. Human resources for health 2014; 12(1): 45.
- ↑ Palmer JJ, Chinanayi F, Gilbert A, et al. Mapping human resources for eye health in 21 countries of sub-Saharan Africa: current progress towards VISION 2020. Human resources for health 2014; 12(1): 44.
- ↑ Jump up to: 9.0 9.1 Jackson DJ, Razai MS, Falama R, et al. The clinical characteristics of patients with glaucoma presenting to Botswana healthcare facilities: an observational study. BMJ open 2014; 4(12).
- ↑ Isawumi MA, Hassan MB, Akinwusi PO, et al. Awareness of and Attitude towards glaucoma among an adult rural population of Osun State, Southwest Nigeria. Middle East African journal of ophthalmology 2014; 21(2): 165.
- ↑ Shrestha MK, Guo CW, Maharjan N, Gurung R, Ruit S. Health literacy of common ocular diseases in Nepal. BMC ophthalmology 2014; 14(1): 2.
- ↑ Ruit S, Tabin G, Wykoff CC. Fighting Global Blindness: Improving World Vision Through Cataract Elimination: American Public Health Association; 2006.
- ↑ Adio AO, Onua AA. Economic burden of glaucoma in Rivers State, Nigeria. Clinical ophthalmology (Auckland, NZ) 2012; 6: 2023.
- ↑ Jump up to: 14.0 14.1 14.2 Liang YB, Wang NL, Rong SS, Thomas R. Initial treatment for primary angle-closure glaucoma in China. Journal of glaucoma 2015; 24(6): 469-73.
- ↑ Jump up to: 15.0 15.1 15.2 Bourne RR. Vision 2020: where are we? Current Opinion in Ophthalmology 2020; 31(2): 81-4.
- ↑ Jump up to: 16.0 16.1 16.2 16.3 Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British journal of ophthalmology 2006; 90(3): 262-7.
- ↑ Jump up to: 17.0 17.1 17.2 Cheng C-Y, Wang N, Wong TY, et al. Prevalence and causes of vision loss in East Asia in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2020; 104(5): 616-22.
- ↑ Jump up to: 18.0 18.1 18.2 Keeffe JE, Casson RJ, Pesudovs K, et al. Prevalence and causes of vision loss in South-east Asia and Oceania in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2019; 103(7): 878-84.
- ↑ Jump up to: 19.0 19.1 19.2 Leasher JL, Braithwaite T, Furtado JM, et al. Prevalence and causes of vision loss in Latin America and the Caribbean in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2019; 103(7): 885-93.
- ↑ Jump up to: 20.0 20.1 20.2 Naidoo K, Kempen JH, Gichuhi S, et al. Prevalence and causes of vision loss in sub-Saharan Africa in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2020.
- ↑ Jump up to: 21.0 21.1 21.2 Nangia V, Jonas JB, George R, et al. Prevalence and causes of blindness and vision impairment: magnitude, temporal trends and projections in South and Central Asia. British Journal of Ophthalmology 2019; 103(7): 871-7.
- ↑ Jump up to: 22.0 22.1 Bourne RR, Jonas JB, Bron AM, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2018; 102(5): 575-85.
- ↑ Jump up to: 23.0 23.1 Cheng C-Y, Wang N, Wong TY, et al. Prevalence and causes of vision loss in East Asia in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2020; 104(5): 616-22.
- ↑ Jump up to: 24.0 24.1 Keeffe JE, Casson RJ, Pesudovs K, et al. Prevalence and causes of vision loss in South-east Asia and Oceania in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2019; 103(7): 878-84.
- ↑ Jump up to: 25.0 25.1 Leasher JL, Braithwaite T, Furtado JM, et al. Prevalence and causes of vision loss in Latin America and the Caribbean in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2019; 103(7): 885-93.
- ↑ Jump up to: 26.0 26.1 Naidoo K, Kempen JH, Gichuhi S, et al. Prevalence and causes of vision loss in sub-Saharan Africa in 2015: magnitude, temporal trends and projections. British Journal of Ophthalmology 2020.
- ↑ Jump up to: 27.0 27.1 Nangia V, Jonas JB, George R, et al. Prevalence and causes of blindness and vision impairment: magnitude, temporal trends and projections in South and Central Asia. British Journal of Ophthalmology 2019; 103(7): 871-7.
- ↑ Jump up to: 28.0 28.1 Kosoko-Lasaki O, Gong G, Haynatzki G, Wilson MR. Race, ethnicity and prevalence of primary open-angle glaucoma. Journal of the National Medical Association 2006; 98(10): 1626.
- ↑ Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. Jama 1991; 266(3): 369-74.
- ↑ Jump up to: 30.0 30.1 Cho H-k, Kee C. Population-based glaucoma prevalence studies in Asians. Survey of ophthalmology 2014; 59(4): 434-47.
- ↑ Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia: a population-based survey in Hövsgöl Province, northern Mongolia. Archives of ophthalmology 1996; 114(10): 1235-41.
- ↑ Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology 2004; 111(9): 1641-8.
- ↑ Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Experimental eye research 2009; 88(4): 837-44.
- ↑ Takamoto M, Araie M. Genetics of primary open angle glaucoma. Japanese journal of ophthalmology 2014; 58(1): 1-15.
- ↑ Williams SE, Carmichael TR, Allingham RR, Hauser M, Ramsay M. The genetics of POAG in black South Africans: a candidate gene association study. Scientific reports 2015; 5(1): 1-7.
- ↑ Casson R, Newland H, Muecke J, et al. Prevalence of glaucoma in rural Myanmar: the Meiktila Eye Study. British journal of ophthalmology 2007; 91(6): 710-4.
- ↑ Jump up to: 37.0 37.1 Cheng J-W, Zong Y, Zeng Y-Y, Wei R-L. The prevalence of primary angle closure glaucoma in adult Asians: a systematic review and meta-analysis. PloS one 2014; 9(7): e103222.
- ↑ Yazdani S, Akbarian S, Pakravan M, Afrouzifar M. Prevalence of angle closure in siblings of patients with primary angle-closure glaucoma. Journal of glaucoma 2015; 24(2): 149-53.
- ↑ Amerasinghe N, Zhang J, Thalamuthu A, et al. The heritability and sibling risk of angle closure in Asians. Ophthalmology 2011; 118(3): 480-5.
- ↑ Kavitha S, Zebardast N, Palaniswamy K, et al. Family history is a strong risk factor for prevalent angle closure in a South Indian population. Ophthalmology 2014; 121(11): 2091-7.
- ↑ Sihota R, Ghate D, Mohan S, Gupta V, Pandey R, Dada T. Study of biometric parameters in family members of primary angle closure glaucoma patients. Eye 2008; 22(4): 521-7.
- ↑ Spaeth G. Angle-closure glaucoma in East Asian and European people. Different diseases? Eye 2007; 21(1): 99-100.
- ↑ Yip JL, Foster PJ. Ethnic differences in primary angle-closure glaucoma. Current opinion in ophthalmology 2006; 17(2): 175-80.
- ↑ Liza-Sharmini AT, Sharina YN, Dolaboladi A, Zaid N, Azhany Y, Zunaina E. Clinical presentation, severity and progression of primary angle closure in malays. The Medical journal of Malaysia 2014; 69(1): 21.
- ↑ Azhany MO. Clinical presentation, severity and progression of primary angle closure in Malay and Chinese patients. Med J Malaysia 2014; 69(6): 245.
- ↑ Jump up to: 46.0 46.1 46.2 Gilbert C, Foster A. Childhood blindness in the context of VISION 2020: the right to sight. Bulletin of the World Health Organization 2001; 79: 227-32.
- ↑ Jump up to: 47.0 47.1 Koay C, Patel D, Tajunisah I, Subrayan V, Lansingh V. A comparative analysis of avoidable causes of childhood blindness in Malaysia with low income, middle income and high income countries. International Ophthalmology 2015; 35(2): 201-7.
- ↑ Moore DB, Tomkins O, Ben-Zion I. A review of primary congenital glaucoma in the developing world. Survey of ophthalmology 2013; 58(3): 278-85.
- ↑ Jump up to: 49.0 49.1 Papadopoulos M, Cable N, Rahi J, Khaw PT. The British infantile and childhood glaucoma (BIG) eye study. Investigative ophthalmology & visual science 2007; 48(9): 4100-6.
- ↑ Yuan F, Qingqing L, Wenyi G, Xinghuai S. Profile of pediatric glaucoma patients in Shanghai Eye, Ear, Nose and Throat Hospital. Chinese Medical Journal 2014; 127(8): 1429-33.
- ↑ Ben-Zion I, Tomkins O, Moore DB, Helveston EM. Surgical results in the management of advanced primary congenital glaucoma in a rural pediatric population. Ophthalmology 2011; 118(2): 231-5. e1.
- ↑ Zagora SL, Funnell CL, Martin FJ, et al. Primary congenital glaucoma outcomes: lessons from 23 years of follow-up. American journal of ophthalmology 2015; 159(4): 788-96. e2.
- ↑ Alsheikheh A, Klink J, Klink T, Steffen H, Grehn F. Long-term results of surgery in childhood glaucoma. Graefe's Archive for Clinical and Experimental Ophthalmology 2007; 245(2): 195-203.
- ↑ Haargaard B, Ritz C, Oudin A, et al. Risk of glaucoma after pediatric cataract surgery. Investigative ophthalmology & visual science 2008; 49(5): 1791-6.
- ↑ Lawrence MG, Kramarevsky NY, Christiansen SP, Wright MM, Young TL, Summers CG. Glaucoma following cataract surgery in children: surgically modifiable risk factors. Transactions of the American Ophthalmological Society 2005; 103: 46.
- ↑ Magnusson G, Abrahamsson M, Sjöstrand J. Glaucoma following congenital cataract surgery: an 18‐year longitudinal follow‐up. Acta ophthalmologica Scandinavica 2000; 78(1): 65-70.
- ↑ Swamy BN, Billson F, Martin F, et al. Secondary glaucoma after paediatric cataract surgery. British journal of ophthalmology 2007; 91(12): 1627-30.
- ↑ Paudyal I, Thapa SS, Paudyal G, et al. Glaucoma at a tertiary referral eye hospital in Nepal. Nepal Journal of Ophthalmology 2011; 3(2): 123-7.
- ↑ Ashaye A, Ashaolu O, Komolafe O, et al. Prevalence and types of glaucoma among an indigenous African population in southwestern Nigeria. Investigative ophthalmology & visual science 2013; 54(12): 7410-6.
- ↑ Asuquo IM, Busuyi HM, Umar KO. The dangers of couching in southwest Nigeria. The Malaysian journal of medical sciences: MJMS 2014; 21(5): 60.
- ↑ Meda N, Bognounou V, Seni E, Daboue A, Sanfo O. Cataract in Burkina Faso: factors of choice between modern and traditional surgical procedures. Medecine tropicale: revue du Corps de sante colonial 2005; 65(5): 473.
- ↑ Omoti A. Complications of traditional couching in a Nigerian local population. West African journal of medicine 2005; 24(1): 7-9.
- ↑ Schémann J-F, Bakayoko S, Coulibaly S. Traditional couching is not an effective alternative procedure for cataract surgery in Mali. Ophthalmic epidemiology 2000; 7(4): 271-83.
- ↑ Ashaye A, Adeoti C. Neovascular glaucoma in a Nigerian African population. East African medical journal 2006; 83(10): 559-64.
- ↑ Brown GC, Magargal LE, Schachat A, Shah H. Neovascular glaucoma: etiologic considerations. Ophthalmology 1984; 91(4): 315-20.
- ↑ Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World journal of diabetes 2015; 6(1): 92.
- ↑ Al-Shamsi HN, Dueker DK, Nowilaty SR, Al-Shahwan SA. Neovascular glaucoma at king khaled eye specialist hospital–etiologic considerations. Middle East African Journal of Ophthalmology 2009; 16(1): 15.
- ↑ Gadia R, Sihota R, Dada T, Gupta V. Current profile of secondary glaucomas. Indian journal of ophthalmology 2008; 56(4): 285.
- ↑ Tenkir A, Solomon B, Deribew A. Glaucoma subtypes in Ethiopian clinic patients. Journal of glaucoma 2013; 22(2): 110-6.
- ↑ Vijaya L, Asokan R, Panday M, et al. The prevalence of pseudoexfoliation and the long-term changes in eyes with pseudoexfoliation in a South Indian population. Journal of Glaucoma 2016; 25(6): e596-e602.
- ↑ Jump up to: 71.0 71.1 Kuper H, Polack S, Mathenge W, et al. Does cataract surgery alleviate poverty? Evidence from a multi-centre intervention study conducted in Kenya, the Philippines and Bangladesh. PloS one 2010; 5(11): e15431.
- ↑ Zimmer Z. Poverty, wealth inequality and health among older adults in rural Cambodia. Social Science & Medicine 2008; 66(1): 57-71.
- ↑ Gupta V, Srinivasan G, Mei S, Gazzard G, Sihota R, Kapoor K. Utility values among glaucoma patients: an impact on the quality of life. British journal of ophthalmology 2005; 89(10): 1241-4.
- ↑ Jampel HD, Schwartz A, Pollack I, Abrams D, Weiss H, Miller R. Glaucoma patients' assessment of their visual function and quality of life. Journal of glaucoma 2002; 11(2): 154-63.
- ↑ Gothwal VK, Bagga DK, Rao HL, et al. Is utility-based quality of life in adults affected by glaucoma? Investigative Ophthalmology & Visual Science 2014; 55(3): 1361-9.
- ↑ Guedes RAP, Guedes VMP, Freitas SM, Chaoubah A. Utility values for glaucoma in Brazil and their correlation with visual function. Clinical Ophthalmology (Auckland, NZ) 2014; 8: 529.
- ↑ Zhou C, Qian S, Wu P, Qiu C. Quality of life of glaucoma patients in China: sociodemographic, clinical, and psychological correlates—a cross-sectional study. Quality of Life Research 2014; 23(3): 999-1008.
- ↑ Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. Jama 2014; 311(10): 1052-60.
- ↑ Braich PS, Lal V, Hollands S, Almeida DR. Burden and depression in the caregivers of blind patients in India. Ophthalmology 2012; 119(2): 221-6.
- ↑ Köberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: a systematic review. BMJ open 2013; 3(11).
- ↑ Wittenborn JS, Rein DB. The cost-effectiveness of glaucoma interventions in Barbados and Ghana. Optometry and vision science: official publication of the American Academy of Optometry 2011; 88(1): 155.
- ↑ Hernández R, Rabindranath K, Fraser C, Vale L, Blanco AA, Burr JM. Screening for open angle glaucoma: systematic review of cost-effectiveness studies. Journal of glaucoma 2008; 17(3): 159-68.
- ↑ Jump up to: 83.0 83.1 83.2 Organization WH. World report on vision. 2019. 2020.
- ↑ Jump up to: 84.0 84.1 84.2 84.3 84.4 Rani PK, Nangia V, Murthy KR, Khanna RC, Das T. Community care for diabetic retinopathy and glaucoma in India: A panel discussion. Indian Journal of Ophthalmology 2018; 66(7): 916.
- ↑ Jump up to: 85.0 85.1 Cook C. Glaucoma in Africa: size of the problem and possible solutions. Journal of glaucoma 2009; 18(2): 124-8.
- ↑ Gyasi M, Amoako W, Adjuik M. Presentation patterns of primary open angle glaucomas in north eastern ghana. Ghana medical journal 2010; 44(1).
- ↑ Jump up to: 87.0 87.1 Abdull MM, Gilbert CC, Evans J. Primary open angle glaucoma in northern Nigeria: stage at presentation and acceptance of treatment. BMC ophthalmology 2015; 15(1): 111.
- ↑ Kyari F, Nolan W, Gilbert C. Ophthalmologists' practice patterns and challenges in achieving optimal management for glaucoma in Nigeria: results from a nationwide survey. BMJ open 2016; 6(10): e012230.
- ↑ Jump up to: 89.0 89.1 Adekoya B, Adepoju F, Moshood K, Balarabe A. Challenges in the management of glaucoma in a developing country; a qualitative study of providers' perspectives. Nigerian Journal of Medicine 2015; 24(4): 315-22.
- ↑ Jump up to: 90.0 90.1 90.2 Delgado MF, Abdelrahman AM, Terahi M, et al. Management Of Glaucoma In Developing Countries: Challenges And Opportunities For Improvement. ClinicoEconomics and Outcomes Research: CEOR 2019; 11: 591.
- ↑ Killeen OJ, Pillai MR, Udayakumar B, et al. Understanding Barriers to Glaucoma Treatment Adherence among Participants in South India. Ophthalmic Epidemiology 2020; 27(3): 200-8.
- ↑ Nayak B, Gupta S, Kumar G, Dada T, Gupta V, Sihota R. Socioeconomics of long-term glaucoma therapy in India. Indian Journal of Ophthalmology 2015; 63(1): 20.
- ↑ Tamrat L, Gessesse GW, Gelaw Y. Adherence to topical glaucoma medications in Ethiopian patients. Middle East African journal of ophthalmology 2015; 22(1): 59.
- ↑ Jump up to: 94.0 94.1 Thomas R. Glaucoma in developing countries. Indian journal of ophthalmology 2012; 60(5): 446.
- ↑ Jump up to: 95.0 95.1 Ting NS, Yim JFL, Ng JY. Different strategies and cost-effectiveness in the treatment of primary open angle glaucoma. ClinicoEconomics and outcomes research: CEOR 2014; 6: 523.
- ↑ Jump up to: 96.0 96.1 96.2 Anand A, Negi S, Khokhar S, et al. Role of early trabeculectomy in primary open-angle glaucoma in the developing world. Eye 2007; 21(1): 40-5.
- ↑ Jump up to: 97.0 97.1 97.2 Quigley HA, Buhrmann RR, West SK, Isseme I, Scudder M, Oliva MS. Long term results of glaucoma surgery among participants in an east African population survey. British Journal of Ophthalmology 2000; 84(8): 860-4.
- ↑ Jump up to: 98.0 98.1 Adekoya BJ, Onakoya AO, Shah SP, Adepoju FG. Surgical output and clinic burden of glaucoma in Lagos, Nigeria. Journal of glaucoma 2014; 23(1): 41-5.
- ↑ Jump up to: 99.0 99.1 Dhalla K, Cousens S, Bowman R, Wood M, Murdoch I. Is beta radiation better than 5 flurouracil as an adjunct for trabeculectomy surgery when combined with cataract surgery? A randomised controlled trial. PloS one 2016; 11(9): e0161674.
- ↑ Ramchandani M. Glaucoma in the developing world. British Medical Journal Publishing Group; 2006.
- ↑ Cabourne E, Clarke JC, Schlottmann PG, Evans JR. Mitomycin C versus 5‐Fluorouracil for wound healing in glaucoma surgery. Cochrane Database of Systematic Reviews 2015; (11).
- ↑ Jump up to: 102.0 102.1 Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. American journal of ophthalmology 2012; 153(5): 804-14. e1.
- ↑ Duman F, Faria B, Rutnin N, et al. Comparison of 3 different releasable suture techniques in trabeculectomy. European journal of ophthalmology 2016; 26(4): 307-14.
- ↑ Venkatesh R, Sengupta S, Robin AL. Mitomycin C–Augmented Trabeculectomy Combined With Single-Site Manual Small-Incision Cataract Surgery Through a Tunnel Flap Technique. The Asia-Pacific Journal of Ophthalmology 2012; 1(3): 142-6.
- ↑ Broadway D, Grierson I, Hitchings R. Racial differences in the results of glaucoma filtration surgery: are racial differences in the conjunctival cell profile important? British Journal of Ophthalmology 1994; 78(6): 466-75.
- ↑ Investigators A. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology 2004; 111(4): 651-64.
- ↑ Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology 2011; 118(3): 443-52.
- ↑ Christakis PG, Kalenak JW, Zurakowski D, et al. The Ahmed Versus Baerveldt study: one-year treatment outcomes. Ophthalmology 2011; 118(11): 2180-9.
- ↑ Kiage D, Gradin D, Gichuhi S, Damji K. Ahmed glaucoma valve implant: experience in East Africa. Middle East African Journal of Ophthalmology 2009; 16(3): 151.
- ↑ Giorgis A. Initial clinical experience of tube-shunt surgery in Ethiopian patients with refractory glaucoma. Ethiop Med J 2012; 50(2): 159-65.
- ↑ Aminlari AE, Scott IU, Aref AA. Glaucoma drainage implant surgery–An evidence-based update with relevance to Sub-Saharan Africa. Middle East African journal of ophthalmology 2013; 20(2): 126.
- ↑ Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin in eyes with and without surgical complications: Results from 2 million consecutive cataract surgeries. J Cataract Refract Surg 2019; 45(9): 1226-33.
- ↑ Mitchell W, Tom L, Durai I, et al. The Effectiveness of Intracameral Moxifloxacin Endophthalmitis Prophylaxis for Trabeculectomy. Ophthalmol Glaucoma 2020.
- ↑ Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology 2008; 115(12): 2167-73. e2.
- ↑ Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology 2009; 116(4): 725-31. e3.
- ↑ Bechrakis N, Dimmer A. Rhegmatogene Netzhautablösung. Der Ophthalmologe 2018; 115(2): 163-78.
- ↑ Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. The Lancet 2016; 388(10052): 1389-97.
- ↑ Tham CC, Kwong YY, Baig N, Leung DY, Li FC, Lam DS. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmology 2013; 120(1): 62-7.
- ↑ Kiddee W, Tantisarasart T, Wangsupadilok B. Neovascular glaucoma: a retrospective review of 5-year experience in Songklanagarind Hospital. J Med Assoc Thai 2012; 95(Suppl 4): S36-S42.
- ↑ Realini T. Selective laser trabeculoplasty for the management of open-angle glaucoma in St. Lucia. JAMA ophthalmology 2013; 131(3): 321-7.
- ↑ Lee JW, Liu CC, Chan JC, Lai JS. Predictors of success in selective laser trabeculoplasty for Chinese open-angle glaucoma. Journal of glaucoma 2014; 23(5): 321-5.
- ↑ Realini T, Olawoye O, Kizor-Akaraiwe N, Manji S, Sit A. The rationale for selective laser trabeculoplasty in Africa. The Asia-Pacific Journal of Ophthalmology 2018; 7(6): 387-93.
- ↑ Gupta V, Agarwal HC. Contact trans-scleral laser cyclophotocoagulation treatment for refractory glaucomas in the Indian population. Indian Journal of Ophthalmology 2000; 48(4): 295.
- ↑ Schwering MS, Kayange P, Klauss V, Kalua K, Spitzer MS. Low-dose transscleral diode laser cyclophotocoagulation (TSCPC) as a potential single treatment for primary open-angle glaucoma (POAG) in Malawi? Graefe's Archive for Clinical and Experimental Ophthalmology 2013; 251(10): 2389-93.
- ↑ Mavrakanas N, Dhalla K, Kapesa I, Alibhai A, Murdoch I. Diode laser transscleral cyclophotocoagulation for the treatment of glaucoma in East Africa. Eye 2013; 27(3): 453-4.
- ↑ Preußner P-R, Ngounou F, Kouogan G. Controlled cyclophotocoagulation with the 940 nm laser for primary open angle glaucoma in African eyes. Graefe's Archive for Clinical and Experimental Ophthalmology 2010; 248(10): 1473-9.
- ↑ Johnstone MA, Shaozhen S, Padilla S, et al. Microscope Real-time Video (MRTV), High-resolution OCT (HR-OCT) & Histopathology (HP) to Assess How Transcleral Micropulse Laser (TML) Affects the Sclera, Ciliary Body (CB), Muscle (CM), Secretory Epithelium (CBSE), Suprachoroidal Space (SCS) & Aqueous Outflow System. Investigative Ophthalmology & Visual Science 2019; 60(9): 2825-.
- ↑ The Commonwealth Fund. What Impact Has COVID-19 Had on Outpatient Visits?. https://www.commonwealthfund.org/publications/2020/apr/impact-covid-19-outpatient-visits. Accessed 2 May 2020.
- ↑ Nair AG, Gandhi RA, Natarajan S. Effect of COVID-19 related lockdown on ophthalmic practice and patient care in India: Results of a survey. Indian J Ophthalmol 2020; 68(5): 725-30.
- ↑ Jump up to: 130.0 130.1 Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Health and Surveillance 2020; 6(2): e18810.
- ↑ Jump up to: 131.0 131.1 Mann DM, Chen J, Chunara R, Testa PA, Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. Journal of the American Medical Informatics Association 2020.
- ↑ Jump up to: 132.0 132.1 Hollander JE, Carr BG. Virtually Perfect? Telemedicine for Covid-19. N Engl J Med 2020; 382(18): 1679-81.
- ↑ Jump up to: 133.0 133.1 Chauhan V, Galwankar S, Arquilla B, et al. Novel coronavirus (COVID-19): Leveraging telemedicine to optimize care while minimizing exposures and viral transmission. Journal of Emergencies, Trauma, and Shock 2020; 13(1): 20.
- ↑ Jump up to: 134.0 134.1 Romano MR, Montericcio A, Montalbano C, et al. Facing COVID-19 in Ophthalmology Department. Curr Eye Res 2020: 1-6.
- ↑ Kalra G, Williams AM, Commiskey PW, et al. Incorporating Video Visits into Ophthalmology Practice: A Retrospective Analysis and Patient Survey to Assess Initial Experiences and Patient Acceptability at an Academic Eye Center. Ophthalmology and Therapy 2020: 1-14.
- ↑ Williams AM, Kalra G, Commiskey PW, et al. Ophthalmology practice during the coronavirus disease 2019 pandemic: The University of Pittsburgh Experience in promoting clinic safety and embracing video visits. Ophthalmology and therapy 2020: 1.
- ↑ Jump up to: 137.0 137.1 137.2 Mohammadpour M, Heidari Z, Mirghorbani M, Hashemi H. Smartphones, tele-ophthalmology, and VISION 2020. International journal of ophthalmology 2017; 10(12): 1909.
- ↑ Jump up to: 138.0 138.1 Stanzel B, Meyer C. Smartphones in ophthalmology: Relief or toys for physicians? Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 2012; 109(1): 8-20.
- ↑ Jump up to: 139.0 139.1 Chhablani J, Kaja S, Shah VA. Smartphones in ophthalmology. Indian journal of ophthalmology 2012; 60(2): 127.
- ↑ Jump up to: 140.0 140.1 Lord RK, Shah VA, San Filippo AN, Krishna R. Novel uses of smartphones in ophthalmology. Ophthalmology 2010; 117(6): 1274-. e3.
- ↑ Jump up to: 141.0 141.1 Labiris G, Panagiotopoulou E-K, Kozobolis VP. A systematic review of teleophthalmological studies in Europe. International journal of ophthalmology 2018; 11(2): 314.
- ↑ Jump up to: 142.0 142.1 Sreelatha OK, Ramesh SV. Teleophthalmology: improving patient outcomes? Clinical ophthalmology (Auckland, NZ) 2016; 10: 285.
- ↑ Jump up to: 143.0 143.1 Zimmer-Galler IE, Zeimer R. Telemedicine in Diabetic Retinopathy Screening. International Ophthalmology Clinics 2009; 49(2): 75-86.
- ↑ Jump up to: 144.0 144.1 Bar-Sela SM, Glovinsky Y. A feasibility study of an Internet-based telemedicine system for consultation in an ophthalmic emergency room. J Telemed Telecare 2007; 13(3): 119-24.
- ↑ Jump up to: 145.0 145.1 Paul PG, Raman R, Rani PK, Deshmukh H, Sharma T. Patient satisfaction levels during teleophthalmology consultation in rural South India. Telemedicine Journal & e-Health 2006; 12(5): 571-8.
- ↑ Jump up to: 146.0 146.1 Labiris G, Fanariotis M, Christoulakis C, et al. Tele-ophthalmology and conventional ophthalmology using a mobile medical unit in remote Greece. Journal of telemedicine and telecare 2003; 9(5): 296-9.
- ↑ Jump up to: 147.0 147.1 Fatehi F, Jahedi F, Tay-Kearney M-L, Kanagasingam Y. Teleophthalmology for the elderly population: A review of the literature. International Journal of Medical Informatics 2020: 104089.
- ↑ Host BKJ, Turner AW, Muir J. Real-time teleophthalmology video consultation: an analysis of patient satisfaction in rural Western Australia. Clinical and Experimental Optometry 2018; 101(1): 129-34.
- ↑ Tan IJ, Dobson LP, Bartnik S, Muir J, Turner AW. Real-time teleophthalmology versus face-to-face consultation: A systematic review. J Telemed Telecare 2017; 23(7): 629-38.
- ↑ Johnson KA, Meyer J, Yazar S, Turner AW. Real-time teleophthalmology in rural Western Australia. The Australian journal of rural health 2015; 23(3): 142-9.
- ↑ Tan JC, Poh EW, Srinivasan S, Lim TH. A pilot trial of tele-ophthalmology for diagnosis of chronic blurred vision. J Telemed Telecare 2013; 19(2): 65-9.
- ↑ Choudhari NS, Chandran P, Rao HL, et al. LVPEI Glaucoma Epidemiology and Molecular Genetic Study: teleophthalmology screening for angle-closure disease in an underserved region. Eye 2019: 1-7.
- ↑ Odden JL, Khanna CL, Choo CM, et al. Telemedicine in long-term care of glaucoma patients. Journal of Telemedicine and Telecare 2020; 26(1-2): 92-9.
- ↑ Kotecha A, Baldwin A, Brookes J, Foster PJ. Experiences with developing and implementing a virtual clinic for glaucoma care in an NHS setting. Clin Ophthalmol 2015; 9: 1915-23.
- ↑ Kim DY, Delori F, Mukai S. Smartphone photography safety. Ophthalmology 2012; 119(10): 2200-1.
- ↑ Kalra G IP. Kalra G, Ichhpujani P. Smartphone-Based Ocular Photography: Pointers for shortening the learning curve and improving image quality. https://glaucomatoday.com/articles/2019-nov-dec/smartphone-based-ocular-photography. Glaucoma Today. Accessed 5 May 2020.
- ↑ Kalra G, Ichhpujani P, Thakur S, Singh RB, Sharma U, Kumar S. A pilot study for smartphone photography to assess bleb morphology and vasculature post-trabeculectomy. International Ophthalmology 2020: 1-8.
- ↑ Kumar N, Francesco B, Sharma A. Smartphone-based Gonio-Imaging: A Novel Addition to Glaucoma Screening Tools. Journal of glaucoma 2019; 28(9): e149-e50.
- ↑ Pujari A, Mukhija R, Chawla R, Phuljhele S, Saxena R, Sharma P. Smartphone-based evaluation of the optic nerve head. Indian journal of ophthalmology 2018; 66(11): 1617-8.
- ↑ Toy BC, Myung DJ, He L, et al. Smartphone-based dilated fundus photography and near visual acuity testing as inexpensive screening tools to detect referral warranted diabetic eye disease. Retina 2016; 36(5): 1000-8.
- ↑ Russo A, Morescalchi F, Costagliola C, Delcassi L, Semeraro F. A novel device to exploit the smartphone camera for fundus photography. Journal of ophthalmology 2015; 2015.
- ↑ Rajalakshmi R, Arulmalar S, Usha M, et al. Validation of smartphone based retinal photography for diabetic retinopathy screening. PloS one 2015; 10(9): e0138285.
- ↑ Haddock LJ, Kim DY, Mukai S. Simple, inexpensive technique for high-quality smartphone fundus photography in human and animal eyes. Journal of ophthalmology 2013; 2013.
- ↑ Kumar S, Wang E-H, Pokabla MJ, Noecker RJ. Teleophthalmology assessment of diabetic retinopathy fundus images: smartphone versus standard office computer workstation. TELEMEDICINE and e-HEALTH 2012; 18(2): 158-62.
- ↑ Russo A, Mapham W, Turano R, et al. Comparison of smartphone ophthalmoscopy with slit-lamp biomicroscopy for grading vertical cup-to-disc ratio. Journal of glaucoma 2016; 25(9): e777-e81.
- ↑ Rodriguez-Una I, Azuara-Blanco A. New technologies for glaucoma detection. The Asia-Pacific Journal of Ophthalmology 2018; 7(6): 394-404.
- ↑ Tsapakis S, Papaconstantinou D, Diagourtas A, et al. Home-based visual field test for glaucoma screening comparison with Humphrey perimeter. Clinical Ophthalmology (Auckland, NZ) 2018; 12: 2597.
- ↑ Mariakakis A, Wang E, Patel S, Wen JC. A smartphone-based system for assessing intraocular pressure. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference 2016; 2016: 4353-6.
- ↑ Rajalakshmi R, Subashini R, Anjana RM, Mohan V. Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye 2018; 32(6): 1138-44.
- ↑ Natarajan S, Jain A, Krishnan R, Rogye A, Sivaprasad S. Diagnostic accuracy of community-based diabetic retinopathy screening with an offline artificial intelligence system on a smartphone. JAMA ophthalmology 2019; 137(10): 1182-8.
- ↑ Rajalakshmi R. The impact of artificial intelligence in screening for diabetic retinopathy in India. Nature Publishing Group; 2019.
- ↑ Jump up to: 172.0 172.1 Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. British Journal of Ophthalmology 2019; 103(2): 167-75.
- ↑ Jump up to: 173.0 173.1 Huang, X., Islam, M.R., Akter, S. et al. Artificial intelligence in glaucoma: opportunities, challenges, and future directions. BioMed Eng OnLine 22, 126 (2023).
- ↑ Yousefi S. Clinical Applications of Artificial Intelligence in Glaucoma. J Ophthalmic Vis Res. 2023 Feb 21;18(1):97-112.
- ↑ Zhang L, Tang L, Xia M, Cao G. The application of artificial intelligence in glaucoma diagnosis and prediction. Front Cell Dev Biol. 2023 May 4;11:1173094.
- ↑ Delgado MF, Abdelrahman AM, Terahi M, Miro Quesada Woll JJ, Gil-Carrasco F, Cook C, Benharbit M, Boisseau S, Chung E, Hadjiat Y, Gomes JA. Management Of Glaucoma In Developing Countries: Challenges And Opportunities For Improvement. Clinicoecon Outcomes Res. 2019 Sep 27;11:591-604.
- ↑ Nunez R, Harris A, Ibrahim O, Keller J, Wikle CK, Robinson E, Zukerman R, Siesky B, Verticchio A, Rowe L, Guidoboni G. Artificial Intelligence to Aid Glaucoma Diagnosis and Monitoring: State of the Art and New Directions. Photonics. 2022 Nov;9(11):810.
- ↑ Haja SA, Mahadevappa V. Advancing glaucoma detection with convolutional neural networks: a paradigm shift in ophthalmology. Rom J Ophthalmol. 2023 Jul-Sep;67(3):222-237.
- ↑ Ruamviboonsuk P, Chantra S, Seresirikachorn K, Ruamviboonsuk V, Sangroongruangsri S. Economic Evaluations of Artificial Intelligence in Ophthalmology. Asia Pac J Ophthalmol (Phila). 2021 Jul 13;10(3):307-316.
- ↑ uang, XM., Yang, BF., Zheng, WL. et al. Cost-effectiveness of artificial intelligence screening for diabetic retinopathy in rural China. BMC Health Serv Res 22, 260 (2022).
- ↑ Timan, T., Mann, Z. (2021). Data Protection in the Era of Artificial Intelligence: Trends, Existing Solutions and Recommendations for Privacy-Preserving Technologies. In: Curry, E., Metzger, A., Zillner, S., Pazzaglia, JC., García Robles, A. (eds) The Elements of Big Data Value. Springer, Cham.
- ↑ Krishnaiah S, Kovai V, Srinivas M, Shamanna BR, Rao GN, Thomas R. Awareness of glaucoma in the rural population of Southern India. Indian journal of ophthalmology 2005; 53(3): 205.
- ↑ Sathyamangalam RV, Paul PG, George R, et al. Determinants of glaucoma awareness and knowledge in urban Chennai. Indian journal of ophthalmology 2009; 57(5): 355.
- ↑ Gasch AT, Wang P, Pasquale LR. Determinants of glaucoma awareness in a general eye clinic. Ophthalmology 2000; 107(2): 303-8.
- ↑ Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology 1996; 103(10): 1661-9.
- ↑ Adekoya BJ, Akinsola FB, Balogun BG, Balogun MM, Ibidapo OO. Patient refusal of glaucoma surgery and associated factors in Lagos, Nigeria. Middle East African journal of ophthalmology 2013; 20(2): 168.
- ↑ Kong XM, Zhu WQ, Hong JX, Sun XH. Is glaucoma comprehension associated with psychological disturbance and vision-related quality of life for patients with glaucoma? A cross-sectional study. BMJ open 2014; 4(5).
- ↑ Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology 2005; 112(6): 953-61. e7.
- ↑ Craven ER, Walters T, Christie WC, et al. 24-month phase I/II clinical trial of bimatoprost sustained-release implant (Bimatoprost SR) in glaucoma patients. Drugs 2020; 80(2): 167-79.
- ↑ Resnikoff S, Lansingh VC, Washburn L, et al. Estimated number of ophthalmologists worldwide (International Council of Ophthalmology update): will we meet the needs? British Journal of Ophthalmology 2020; 104(4): 588-92.
- ↑ Dean W, Gichuhi S, Buchan J, et al. Survey of ophthalmologists-in-training in Eastern, Central and Southern Africa: A regional focus on ophthalmic surgical education. Wellcome open research 2019; 4.
- ↑ Dean WH, Murray NL, Buchan JC, Golnik K, Kim MJ, Burton MJ. Ophthalmic Simulated Surgical Competency Assessment Rubric for manual small-incision cataract surgery. Journal of Cataract & Refractive Surgery 2019; 45(9): 1252-7.
- ↑ Orbis Cybersight: A global community of learning sharing and practice. https://cybersight.org/. [Accessed on 7/15/20].
- ↑ Jump up to: 194.0 194.1 Du Toit R, Faal HB, Etya’ale D, et al. Evidence for integrating eye health into primary health care in Africa: a health systems strengthening approach. BMC health services research 2013; 13(1): 102.
- ↑ Mehta P, Shenoy S. Infinite vision: how Aravind became the world's greatest business case for compassion: Berrett-Koehler Publishers; 2011.
- ↑ Kappal R, Mehndiratta A, Anandaraj P, Tsanas A. Current impact, future prospects and implications of mobile healthcare in India. Central Asian journal of global health 2014; 3(1).