Foldable Capsular Vitreous Body
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Foldable capsular vitreous body (FCVB) is a novel vitreous substitute that was first developed by Zhongshan Ophthalmic Center, Sun Yat-sen University in China in 2008.[1] It supplemented the preexisting artificial vitreous substitutes such as inert gases, silicone oil (SO), heavy SO, and hydrogels used in cases of ocular pathology that require the removal of the vitreous. It has been reported to be an effective substitute with possibly fewer complications compared to SO.[2]
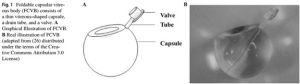
The FCVB is composed of a thin (30 µm) vitreous-shaped capsule, a drain tube, and a valve. (Fig-1)[3] It is made of tailor-made modified liquid silicone rubber.[1] After folded implantation into the vitreous cavity, balanced salt solution (BSS), physiological balanced solution, or SO are injected into the capsule, which is then inflated in order to support the retina and regulate the intraocular pressure (IOP) via the tube–valve system.[4]
While inert gases, SO, heavy SO, and hydrogels are other options for artificial vitreous substitution, the use of FCVB may offer several potential advantages, including better visual and anatomical outcomes, fewer IOP fluctuations, and less complications, especially in complex vitreoretinal conditions compared to SO.[3][5] [6]
Patient Selection
Indications
The FCVB may be used intra-ocularly as a tamponade or extra-ocularly as a macular or scleral buckle.[7] [8] Therefore, FCVB could be used to treat different ocular conditions, including:
- Severe ocular trauma such as perforating and penetrating injuries, severe blunt trauma, and those leading to atrophia bulbi, retinal and choroidal detachments, as well as endophthalmitis.[2]
- SO dependent eyes[7][9] [10] [11]
- Severe proliferative vitreoretinopathy (PVR)[12]
- Recurrent retinal detachment (RD) after SO removal[12] [13] [14]
- Primary failure of RD surgery using SO, C3F8, or heavy SO tamponade[2][15]
- Simple and complex rhegmatogenous RD[16] [17]
- Highly myopic eyes with foveoschisis and posterior staphyloma[8]
Advantages
Many pros for the use of FCVB have been reported in the literature including the following:
- FCVB could be used intraocularly as a tamponade or extra-ocularly as a macular/scleral buckle for various retinal conditions.[7] [8]
- Better postoperative IOP control. Many clinical trials reported improved or maintained IOP postoperatively.[7] [9][18] [19] For optimal IOP outcomes, SO can also be extracted through the FCVB's drainage valve, or the capsule can be reinjected with normal saline and/or SO.[1]
- High retinal reattachment rates by providing a 360° arc pressure effect.[2][12] [13][18]
- Isolating the intraocular tamponade (such as SO) from the retina by the capsule, thus preventing its migration into the surrounding structures[2] [12] [13] [18] which decreases the odds of intraocular tamponade-related complications.[3]
- Patients are not obligated to keep a specific position including the prone position postoperatively.[2]
- It could be used as an intravitreal drug delivery system. Liu Y et al[20] used FCVB to provide sustained release of dexamethasone sodium phosphate and showed promising results.
- Improvement in the best corrected visual acuity (BCVA). In many clinical trials[3][7], FCVB showed promising results by improving final postoperative BCVA, however, the severity of initial injury governs this improvement.
- FCVB implantation results in minor refractive changes compared to other substitutes. On the other hand, Stefansson et al.[21] reported a 9.30 D hyperopic shift in human eyes with SO substitution, while in the Gullstrand-Emsley schematic eye[22], SO tamponade led to higher refractive shifts (+ 8.71 D) compared to BSS-filled FCVB (− 0.338 D).
- Significant improvement in the mental health of patients with severe ocular trauma following FCVB implantation. Shao et al.[14] reported a significant reduction in depression, anxiety, and interpersonal sensitivity rates following FCVB implantation.
Surgical Technique
Operative Procedure
Zhang X et al[9] reported the steps of FCVB implantation as the following:
1. Preoperatively, a customized FCVB could be prepared according to the axial length of the globe.
2. As with all vitreoretinal surgery, the face, eyelids, and conjunctiva are prepared with appropriate antiseptic techniques, and the drapes are applied. A plastic adhesive drape is placed over the opened lids so that the drape adheres to the margins of the eyelids that are held securely in place with the eyelid speculum.
3. A standard 3-port pars plana vitrectomy is conducted to completely remove the vitreous and treat the underlying pathology.
4. An approximately 4 mm incision is performed in the sclera for the FCVB implantation site at either the 4 or 8 o’clock position 4 mm away from the corneal limbus.
5. The capsular integrity of the FCVB is checked, and you may test the air tightness of the FCVB under ddH2O
6. A FCVB is then properly folded and implanted into the vitreous cavity through the incision with a push injector. The concave lens surface of the FCVB should be placed facing the lens. If there is any tilting, the position of the FCVB capsule is adjusted with an iris repositor.
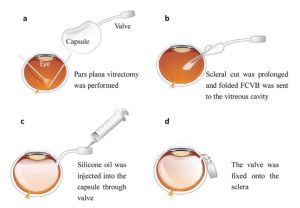
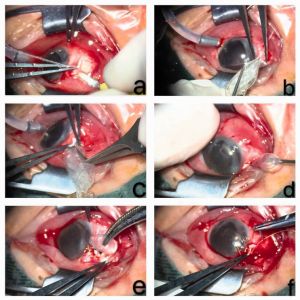
7. Subsequently, SO or saline is slowly injected into the capsule through the drainage valve with a syringe until the IOP is optimal, while the status of the retina and color of the optic disc are observed. (Fig-2 and Fig-3)[23] [24]
8. The scleral incision is sutured, and the drainage tube of the FCVB is ligated and fixed to the sclera.
Complications
Despite that the clinical trials reported fewer complication rates following FCVB implantation compared to other substitutes[3], many complications are reported in the literature[2] [7] [8] [9] [12] [13] [15] [16] [17] [19] [25]:
- Conjunctival hyperemia and/or chemosis
- Epiphora
- Foreign body sensation and/or pain
- Diplopia with some patients having persistent abduction limitation (See https://eyewiki.aao.org/Basic_Approach_to_Diplopia for detailed management approach.)
- Corneal opacity, corneal degeneration and/or even corneal endothelial injury
- Ocular inflammation
- Shallowing of the anterior chamber
- Hyphemia
- Uncomplicated hyphemas should be managed conservatively, with an eye shield, limited activity, and head elevation. Medical treatment for hyphema includes topical corticosteroids (systemic for severe cases), topical cycloplegic agents are also useful for patients with significant ciliary spasm or photophobia. In the setting of IOP elevation, topical aqueous suppressants are first line agents for pressure management (beta-blockers and alpha-agonists). Surgical intervention may be indicated in the setting of uncontrolled glaucoma, corneal blood staining, the persistence of a large or total hyphema, and active bleeding in the anterior chamber.[26] (See https://eyewiki.aao.org/Hyphema for detailed management approach.)
- Possibility of a capsular break during surgery, and/or drainage tube exposure after surgery
- Post-vitrectomy hypotony
- Postoperative hypotony is considered a major concern because it could lead to maculopathy, choroidal detachment, and suprachoroidal hemorrhage. Even more seriously, however, hypotony may imply inadequate closure of sclerotomies, increasing the risk for postoperative endophthalmitis.[27] The management of hypotony depends on the underlying cause. Generally, it includes observation, medical and/or surgical. Medical management lines include topical, local and/or systemic corticosteroids, and intracameral injection of ophthalmic viscosurgical device (OVD). The surgical treatment of ocular hypotony is instituted when the conservative approach has not been successful. This includes Ab Interno partial tube ligation.[28] The routine use of full tamponade could protect the eye from leakage and hypotony. Avoiding, or at least minimizing, the use of intravitreal triamcinolone might lower the incidence of hypotony. Avoiding the use of sutureless vitrectomy in reoperations could further decrease the risk of hypotony.[29]
- Postoperative High IOP:
- The majority of patients without a previous history of ocular hypertension or glaucoma can tolerate a transient postoperative rise in IOP, with no evident effect on visual function.[30] If therapy is needed to control IOP elevation, most cases can be managed medically with antiglaucoma medications. Occasionally, surgical intervention is needed to relieve extremely high IOPs, and the procedure is chosen carefully based on the mechanism of IOP elevation and this which includes anterior chamber paracentesis, laser iridotomy, laser iridoplasty, and laser membranectomy, and even trabeculectomy or placement of glaucoma drainage device.[31] (see https://eyewiki.aao.org/Elevated_Intraocular_Pressure_Associated_with_Retinal_Procedures for further detailed management.)
- Suprachoroidal hemorrhage (SCH)
- The first step in the management of SCH is early recognition, whether it be intraoperative or postoperative. If intraoperative SCH is suspected, immediate tamponade of bleeding vessels via direct pressure or closure of all incisions should be performed to prevent further hemorrhage and subsequent expulsion of intraocular contents.[32] [33] If SCH occurs postoperatively, the management approach is controversial. There is evidence for both non-surgical and surgical intervention. Increased IOP should be treated with a topical beta blocker. Ocular inflammation should be addressed with topical steroids. Ocular pain should be managed with topical cycloplegic agents and oral analgesics. If the SCH does not resolve with medical management or severe ocular pain persists, then reoperation may be considered. Other indications for surgery include uncontrolled intraocular pressure, appositional, or "kissing" choroidals, and retinal detachment.[34] (see https://eyewiki.aao.org/Suprachoroidal_Hemorrhage for detailed management approach.)
Although endophthalmitis can occur following any intraocular procedure, no cases of early or delayed endophthalmitis were reported in association with FCVB implantation.[23]
Summary
Foldable capsular vitreous body is a novel vitreous substitute that has been recently introduced to treat various advanced vitreoretinal conditions including severe ocular trauma, complicated RD, and proliferative vitreoretinopathy. Trials have reported that FCVB showed good visual outcomes, few IOP fluctuations, and a good safety profile. However, more prospective multicenter comparative studies are needed to shed further light on the value of this technique.
References
- ↑ 1.0 1.1 1.2 Gao, Q., et al., A new strategy to replace the natural vitreous by a novel capsular artificial vitreous body with pressure-control valve. Eye (Lond), 2008. 22(3): p. 461-8.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Lin, X., et al., Preliminary efficacy and safety of a silicone oil-filled foldable capsular vitreous body in the treatment of severe retinal detachment. Retina, 2012. 32(4): p. 729-41.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Abu Serhan, H., et al., Foldable capsular vitreous body indications, complications, and outcomes: a systematic review. Graefe's Archive for Clinical and Experimental Ophthalmology, 2023.
- ↑ Wang, P., et al., Biocompatibility and retinal support of a foldable capsular vitreous body injected with saline or silicone oil implanted in rabbit eyes. Clin Exp Ophthalmol, 2012. 40(1): p. e67-75.
- ↑ Chen, J., et al., Clinical device-related article evaluation of morphology and functions of a foldable capsular vitreous body in the rabbit eye. J Biomed Mater Res B Appl Biomater, 2011. 97(2): p. 396-404.
- ↑ Feng, S., et al., A novel vitreous substitute of using a foldable capsular vitreous body injected with polyvinylalcohol hydrogel. Sci Rep, 2013. 3: p. 1838.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Xu, X., et al., Outcomes of a Foldable Capsular Vitreous Body Implantation: A Retrospective Study. Dis Markers, 2021. 2021: p. 6575195.
- ↑ 8.0 8.1 8.2 8.3 Liu, B., et al., MACULAR BUCKLING USING A THREE-ARMED SILICONE CAPSULE FOR FOVEOSCHISIS ASSOCIATED WITH HIGH MYOPIA. Retina, 2016. 36(10): p. 1919-26.
- ↑ 9.0 9.1 9.2 9.3 Zhang, X., et al., Study on the effectiveness and safety of Foldable Capsular Vitreous Body implantation. BMC Ophthalmol, 2019. 19(1): p. 260.
- ↑ Jiang, H., et al. Clinical Application of Foldable Capsular Vitreous Bodies in the Treatment of Severe Ocular Trauma and Silicone Oil Dependent Eyes. 2021.
- ↑ Liu, N., et al., Preliminary clinical application of foldable capsular vitreous body in severe silicone oil-dependent eyes. Ann Palliat Med, 2021. 10(10): p. [[1]].
- ↑ 12.0 12.1 12.2 12.3 12.4 Zhang, Z., et al., Comparison of Viscoelastic Substance Injection Versus Air Filling in the Anterior Chamber During Foldable Capsular Vitreous Body (FCVB) Implant Surgery: A Prospective Randomized Controlled Trial. Adv Ther, 2021. 38(9): p. [[2]].
- ↑ 13.0 13.1 13.2 13.3 Chen, S., et al., Reattachment After Foldable Capsular Vitreous Body Implantation in Severe Retinal Detachment Eyes. Transl Vis Sci Technol, 2021. 10(11): p. 8.
- ↑ 14.0 14.1 Shao, Z., et al., Psychological Effects of Foldable Capsular Vitreous Body Implantation in Patients with Impending Eye Atrophy. 2022, Research Square.
- ↑ 15.0 15.1 Lin, X., et al., Evaluation of the flexibility, efficacy, and safety of a foldable capsular vitreous body in the treatment of severe retinal detachment. Invest Ophthalmol Vis Sci, 2011. 52(1): p. 374-81.
- ↑ 16.0 16.1 Wang, P., et al., Comprehensive analysis of inflammatory immune mediators of the intraocular fluid aspirated from the foldable capsular vitreous body filled-eyes. PLoS One, 2012. 7(10): p. e46384.
- ↑ 17.0 17.1 Zhang, B., et al., A PILOT CLINICAL STUDY OF TREATING RHEGMATOGENOUS RETINAL DETACHMENT BY SILICONE RUBBER BALLOON SCLERAL BUCKLING. Retina, 2020. 40(10): p. 1918-1928.
- ↑ 18.0 18.1 18.2 Zhang, C., et al., SILICONE OIL-FILLED FOLDABLE CAPSULAR VITREOUS BODY VERSUS SILICONE OIL ENDOTAMPONADE FOR TREATMENT OF NO LIGHT PERCEPTION AFTER SEVERE OCULAR TRAUMA. Retina, 2022. 42(3): p. 553-560.
- ↑ 19.0 19.1 Li, M., et al., FOLDABLE CAPSULAR VITREOUS BODY IMPLANTATION FOR COMPLICATED RETINAL DETACHMENT CAUSED BY SEVERE OCULAR TRAUMA. Retina, 2022. 42(8): p. 1512-1519.
- ↑ Liu, Y., et al., Sustained mechanical release of dexamethasone sodium phosphate from a foldable capsular vitreous body. Invest Ophthalmol Vis Sci, 2010. 51(3): p. 1636-42.
- ↑ Stefánsson, E., et al., Refractive changes from use of silicone oil in vitreous surgery. Retina, 1988. 8(1): p. 20-3.
- ↑ Gao, Q., et al., Refractive shifts in four selected artificial vitreous substitutes based on Gullstrand-Emsley and Liou-Brennan schematic eyes. Invest Ophthalmol Vis Sci, 2009. 50(7): p. 3529-34.
- ↑ 23.0 23.1 23.2 Lin, X., et al., Three-Year Efficacy and Safety of a Silicone Oil-Filled Foldable-Capsular-Vitreous-Body in Three Cases of Severe Retinal Detachment. Transl Vis Sci Technol, 2016. 5(1): p. 2.
- ↑ 24.0 24.1 Zeng, B., et al., Foldable capsular vitreous body implantation for treatment of traumatic retinal detachment: two case reports. J Int Med Res, 2021. 49(2): p. [[3]].
- ↑ Deng, J., et al., Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture. Int J Ophthalmol, 2021. 14(12): p. 1935-1940.
- ↑ Wilson, F.M., Traumatic hyphema. Pathogenesis and management. Ophthalmology, 1980. 87(9): p. 910-9.
- ↑ Chen, E., 25-Gauge transconjunctival sutureless vitrectomy. Curr Opin Ophthalmol, 2007. 18(3): p. 188-93.
- ↑ Okonkwo ON, T.K. Ocular Hypotony. 22/02/2023 [cited 2023 28/04/2023]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK582144/.
- ↑ Bamonte, G., M. Mura, and H. Stevie Tan, Hypotony after 25-gauge vitrectomy. Am J Ophthalmol, 2011. 151(1): p. 156-60.
- ↑ Tranos, P., G. Bhar, and B. Little, Postoperative intraocular pressure spikes: the need to treat. Eye (Lond), 2004. 18(7): p. 673-9.
- ↑ Han, D.P., et al., Mechanisms of intraocular pressure elevation after pars plana vitrectomy. Ophthalmology, 1989. 96(9): p. 1357-62.
- ↑ Jiang, H., et al., Risk Factors and Treatments of Suprachoroidal Hemorrhage. Biomed Res Int, 2022. 2022: p. 6539917.
- ↑ Liu, T., et al., Visual and Anatomic Outcomes of Suprachoroidal Hemorrhage: A Systematic Review and Meta-Analysis. Ophthalmol Retina, 2023.
- ↑ Learned, D. and D. Eliott, Management of Delayed Suprachoroidal Hemorrhage after Glaucoma Surgery. Semin Ophthalmol, 2018. 33(1): p. 59-63.

