EyeWatch Implant
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Glaucoma is the leading cause of irreversible blindness worldwide with elevated intraocular pressure (IOP) being the most important risk factor for glaucoma development and progression.[1] Currently, the two most used glaucoma drainage devices (GDDs) are the Baerveldt glaucoma implant (BGI) (Abbott Medical Optics Inc., Santa Ana, CA) and the Ahmed glaucoma valve (AGV) (New World Medical Inc., Rancho Cucamonga, CA).[2] The eyeWatch implant (eW) (Rheon Medical, Switzerland) is a novel device in glaucoma surgery aiming to control aqueous flow through an external, non-invasive magnetic control unit.[3]
Device
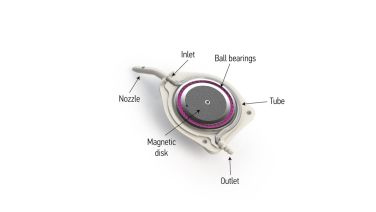
The eW is the world's first adjustable GDD and is unique in its design (Figure 1). It consists of an internal rotating magnet disk, a deformable silicone tube, a bearing system, a nozzle, and an outlet for the adjustable flow of aqueous humor. The device dimensions are 6.5 mm x 5.8 mm with 0.8 mm thickness and a chamber entry size of 25 gauge. The eW is compatible with the eyePlate silicone drainage plate (Figure 2) and other valveless shunts such as the BGI.[4]
The eW is designed to be used in conjunction with the eyeWatch Pen (eWP) that acts as the external control unit for the eW implant. The eWP (Figure 3) consists of a compass on one end to measure the eW's position and a magnet on the other to adjust the opening and closing of the deformable tube. The eW can be set from 0 (fully open) to 6 (fully closed). The sterile eWP is used intra-operatively after eW implantation to set the device to 5-6 to avoid early hypotony. The office eWP is used for post-operative clinic adjustment.[4]
Outcomes
The safety and efficacy of the eW were initially evaluated by Roy and colleagues[2] in a multicentric, prospective, noncomparative clinical trial. They enrolled 15 patients with refractory glaucoma with an IOP ≥ 20 mm Hg after multiple failed glaucoma surgeries. Patients received an eW implant used in conjunction with a BGI. The mean follow-up time was 15.6 ± 3.5 months. The primary outcome was the surgical success rate which was defined as an IOP ≤ 18 mm Hg but ≥ 6 mm Hg and an IOP reduction of > 20% from baseline without (complete) or with (qualified) glaucoma medications. Secondary outcomes included the mean IOP, visual acuity, number of antiglaucoma medications, and number and type of complications. At the last follow-up, the complete success rate (without antiglaucoma medication) was 40%, and the overall success rate (with or without antiglaucoma medication) was 93%. There was a statistically significant reduction in the mean IOP from 26.2 ± 6.8 mm Hg at baseline to 11.9 ± 2.8 mm Hg at 12 months postoperative visit (P < 0.001). The mean number of antiglaucoma medications was significantly decreased from 3.0 ± 0.7 pre-surgery to 0.8 ± 0.9 at the last follow-up visit (P < 0.001). Complications were limited to incomplete wound closure, with no complications related to the device's functioning.[2]
In a retrospective study by Roy and colleagues[5], they compared the eW device and the AGV in refractory glaucoma. This study included 21 patients with a mean age of 73.8 ± 8.4 years at the time of surgery. The mean follow-up time was 13.2 ± 3.4 months. One group included patients who had an AGV implanted, while the other group (eW-B) included patients receiving an eW connected to a BGI. For AGV group, the complete (without antiglaucoma medication) and overall (with antiglaucoma medication) success rates, defined as an IOP between 5-16 mm Hg and an IOP reduction of > 20% from baseline, were 50% and 58%, respectively. For group eW-B, the complete and overall success rates were 67% and 89%, respectively. They reported a statistically significant mean IOP reduction from 24.8 ± 9.0 mm Hg pre-surgery to 13.8 ± 3.6 mm Hg for group AGV and 27.3 ± 7.0 mm Hg to 12.8 ± 2.4 mm Hg for group eW-B, respectively (P<0.05). The mean number of antiglaucoma medications was significantly reduced from 3.0 ± 0.7 pre-surgery to 0.3 ± 0.7 at last follow-up for AGV group and 2.9 ± 0.8 pre-surgery to 0.2 ± 0.4 for eW-B group, respectively (P<0.05). The complication rate was 0% in the eW group compared to 25% in the AGV group. The failure rate in the eW group was 11% compared to 42% in the AGV group.[5]
Detorakis and colleagues[6] evaluated the eW device with an injectable perforated tube rather than implanting a plated valve. The study included three blind, painful eyes of three patients. In all cases, the eW was placed in a standard fashion. However, instead of placing a plated valve, the surgeons inserted a perforated 4-cm-long silicone tube connected to the eW device. The pre-operative IOP was 45 mm Hg, 36 mm Hg, and 42 mm Hg for patients 1, 2, and 3, respectively. Patient 1 and 2 surgeries were successful, with IOP < 15 mm Hg for 12 months and 6 months, respectively. Patient 3's eW implantation was complicated due to increased scar tissue, and the tube had to be cut 1.5 cm to 2.5 cm in length. At 6-month post-operative visit, patient 3's IOP was 40 mm Hg despite maximal topical medications and maximal eW opening.[6]
A recent case study by Elahi and colleagues[7] described the implantation of an eW device attached to a BGI to manage refractory hypotony following traditional BGI surgery. In their case, an 83-year-old white male suffering from longstanding glaucoma underwent initial BGI implantation. However, after 3 months of refractory hypotony, the decision was made to implant an eW-rescue and connect it to the already implanted BGI. On day 1 post-operatively, IOP was 22 mm Hg, and the eW was opened to 5/6 using the eWP to increase aqueous flow. On day 4, IOP decreased to 3 mm Hg, and the eW was closed again to 6/6. IOP then increased to 6 mm Hg at 1 week, 11 mm Hg at 4 weeks, 12 mm Hg at 6 weeks, and 13 mm Hg at 10 weeks without medication. The eW was reopened to 5/6 and remained between 8- and 12-mm Hg over the next 6 months.[7] Roy and Mermoud found that the device effectively lowered intraocular pressure over a 2-year time frame in patients with history of glaucoma surgery.[8]
Conclusion
The eW is the world's first adjustable device for treating glaucoma.[4] Initial studies show that the device effectively lowers IOP safely with fewer post-operative hypotony complications. However, further studies may be needed to adjust implantation techniques.
References
- ↑ Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet. 2017;309:2183–2193.
- ↑ Jump up to: 2.0 2.1 2.2 Roy S, Villamarin A, Stergiopulos C, et al. Initial Clinical Results of the eyeWatch: a New Adjustable Glaucoma Drainage Device Used in Refractory Glaucoma Surgery. J Glaucoma. 2019;28(5):452-458. doi:10.1097/IJG.0000000000001209
- ↑ Detorakis ET, Villamarin A, Roy S, et al. eyeWatch™ System Combined with Non-plated Intraorbital Tube Insertion for the Management of Refractory Glaucoma: A Case Series. J Curr Glaucoma Pract. 2020;14(2):64-67. doi:10.5005/jp-journals-10078-1276
- ↑ Jump up to: 4.0 4.1 4.2 EyeWatch. (2022, May 26). https://rheonmedical.com/products/eyewatch/#
- ↑ Jump up to: 5.0 5.1 Roy S, Villamarin A, Stergiopulos C, Bigler S, Stergiopulos N, Wachtl J, Mermoud A, Kniestedt C. Comparison Between the eyeWatch device and the Ahmed Valve in Refractory Glaucoma. J Glaucoma. 2020 May;29(5):401-405. doi: 10.1097/IJG.0000000000001471. PMID: 32097256.
- ↑ Jump up to: 6.0 6.1 Detorakis ET, Villamarin A, Roy S, Bigler S, Bontzos G, Stergiopulos C, Stergiopulos N. eyeWatch™ System Combined with Non-plated Intraorbital Tube Insertion for the Management of Refractory Glaucoma: A Case Series. J Curr Glaucoma Pract. 2020 May-Aug;14(2):64-67. doi: 10.5005/jp-journals-10078-1276. PMID: 33304062; PMCID: PMC7695936.
- ↑ Jump up to: 7.0 7.1 Elahi S, Bravetti GE, Gillmann K, Villamarin A, Meeus L, Stergiopoulos N, Mansouri K, Mermoud A. EyeWatch Rescue of Refractory Hypotony After Baerveldt Drainage Device Implantation: Description of a New Technique. J Glaucoma. 2020 Feb;29(2):e7-e10. doi: 10.1097/IJG.0000000000001417. PMID: 31821180.
- ↑ Roy S, Mermoud A. Efficacy and Safety of an Adjustable Glaucoma Drainage Device (eyewatch System) for Refractory Glaucoma: A 2-Year Prospective Study. J Glaucoma. 2023 Nov 3. doi: 10.1097/IJG.0000000000002334. Epub ahead of print. PMID: 37974333.