Dysthyroid Optic Neuropathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Dysthyroid Optic Neuropathy
Disease
Dysthyroid optic neuropathy (DON) is an optic nerve dysfunction and one of the severe complications of thyroid eye disease (TED). Predisposing causes includes compressive(CON), ischemic, optic nerve stretch neuropathy or mixed etiology, Untreated and if long standing it may result in permanent visual loss.[1] [2] [3] [4]Because of its potential reversibility with prompt intervention medically, surgically or in combination, accurate and prompt diagnosis and appropriate management are important.
Etiology
TED, also termed Graves Orbitopathy(GO), is an autoimmune disease initiated by several antibodies against thyrotropin (TSH) receptors and adjacent IGF1-receptors on the surface of orbital pluripotential fibrocytes.[5] Binding of these receptors leads to adipogenesis, activation of T-lymphocytes with release of cytokines and initiation of inflammation, and deposition of glycosaminoglycans in orbital fat and muscle.[5][6] Two main types of TED could be categorized. While a majority of TED patients have primarily fat expansion and levator muscle scarring (“adipogenic” phenotype or Type I disease) which rarely leads to DON in cases of nerve stretching from severe proptosis.[7] The other third have a “myopathic” phenotype with enlargement of EOMs and may develop secondary congestive soft tissue signs, ocular motility disruption, and compression of the optic nerve or develop fibrosis in one more muscles.8 In reality, most patients have variable combinations of the above two processes.
Risk Factors
TED affects women more frequently than men with a female to male ratio of 4:1.[8] However, DON is seen more frequently in men with older age, diabetes mellitus and smoking being risk factors.[3][9] [10] Compressive mechanism of DON occurs more commonly in the myopathic subtype. The most common risk factors for this type of optic neuropathy (ON) include smoking [10][11] immune response stimulators (such as intermittent infections, local surgery, and other trigger factors), and comorbidities such as diabetes mellitus.[12] Treatment of hyperthyroidism with radioactive iodine not only increases the risk of progression of TED up to 40% but also the risk of DON.[4]
Epidemiology
DON occurs in 5–8% of all cases of TED. According to the demographic findings of several previous studies, similar to myopathic TED, DON is associated with older age and a higher male-to-female ratio. [2] [3] [4]
Pathophysiology
Several mechanisms have been proposed for the pathogenesis of DON. The most accepted mechanism is compression of optic nerve (CON) by enlarged EOMs at the orbital apex which disturbs axoplasmic flow.[13] The second is from optic nerve stretch from severe proptosis with disruption of the axonal function and blood flow of the optic nerve. This occurs much less frequently than the compressive mechanism.[14] [15] The third mechanism is inflammation which causes optic neuritis. This is supported by the benefit of anti-inflammatories such as corticosteroids for many cases of DON. [13] Occasionally significant weight gain with Type I disease resulting in increased orbital pressure may also predispose to optic neuropathy. In reality, patients may have variable combinations of the above mechanisms.
Diagnosis
In general, diagnosis of DON is made when decreased visual function is related to compromised optic nerve function secondary to TED .[3][16] DON is thus diagnosed based on clinical findings, optic nerve dysfunction studies on automated perimetry, and confirmed by imaging such as CT scans or MRI demonstrating apical optic nerve compression or ON stretch. In some cases, visual-evoked potentials (VEP) may be helpful to provide additional data to support diagnosis. However it is not considered among the first line workup, nor are abnormal results an early finding in DON.
History and Clinical Features
Patients often present with a history of underlying hyperthyroidism under medical treatment, not always under poor control. Patients often also confirm a recent precipitating event – deterioration in general health, personal stress, and very ofter smoking or exposure to tobacco.
The most common presenting symptoms include blurring of central vision and desaturation of colors.[1] [2] [3] [4][17]A relative afferent pupil defect (RAPD) may be noticed in asymmetric cases. Absent RAPD could happen when both eyes have similar vision loss.[1] [16]Optic disc changes such as edema or hyperemia may be detected.[1] [16] In myopathic phenotype, most patients with DON complain about diplopia due to restricted motility. Congestive soft tissue signs are also evident.[1] [3] [4][18][17]
Clinical features of DON include reduced visual acuity (80%), decreased color vision (77%), visual field defect (71%), typically inferior to begin with, often associated with reduced ocular motility (70%) and rarely optic disc swelling (20%).[19]
76% of cases of DON are bilateral.[3]50% to 70% of cases of confirmed DON have best corrected visual acuity of 20/40 or better. These patients may have a greater rise of IOP in upgaze (> 9 mmHg), congestive orbitopathy, tight orbital septum, resistance to retropulsion, and an aching discomfort in the orbit.[20]
Proptosis is not a remarkable finding in compressive DON and some authors believe that tight eyelids may limit anterior movement of the globe and lead to diffuse elevation of orbital tension.[1] [2][10] [21]Conversely, it has been shown that proptosis is not correlated with occurrence of DON.
Other clinical features include a greater rise of IOP in up gaze (> 9 mmHg), congestive orbitopathy from tight orbital septum, resistance to retropulsion with an aching discomfort in the orbit.[20] In the myopathic phenotype, patients have associated diplopia due to restricted motility. Congestive soft tissue signs are also evident. [1] [3] [4] [18] [17]
Optic disc changes such as edema or hyperemia may be detected. [1][16]
These above clinical findings are supported by variable degrees of TED and orbital apex crowding on orbital CT scan. Severe apical crowding is a good predictor of DON, with a sensitivity of 62% and specificity of 91%.[3][16][22]
Diagnostic procedures
Psychophysiologic tests
Amongst various ancillary tests, automated perimetry is the most available and commonly performed.
Visual field
Central and paracentral scotomas occur in most patients with DON. Inferior partial or complete arcuate and altitudinal defects, generalized constriction and enlarged blind spot are detected in 70%.[11][21]
Color desaturation- Red
In patients with previously normal color vision, asymmetric optic nerve neuropathy may present as color desaturation in the worse eye. If symmetric and delayed onset, however, the desaturation may not be obvious.
Relative Afferent Pupillary Defect (RAPD)
This is a simple but very useful finding detecting optic neuropathy. However, if there is symmetrical optic nerve dysfunction , an RAPD would be absent
Visual evoked potentials
Increased latency and decreased amplitude of VEP waves are recorded in ¾ of DON cases.[1][23]VEP can be useful when clinical exams and visual field results are equivocal. This may however represent a late stage of the disease, not universally available and often technique dependent.
Optical coherence tomography (OCT)
Thinning of the retinal nerve fiber layer and macular ganglion cell complex could be seen by means of OCT and this finding correlates with patients' visual function.[24] This finding is an objective way to measure atrophy or thinning prior to any fundus abnormality.
Imaging Studies
Computerized tomographic (CT) and magnetic resonance imaging (MRI)
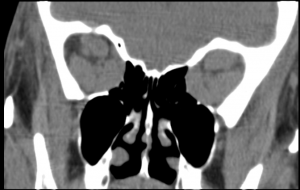
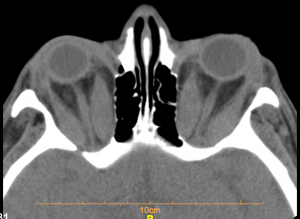
CT and MRI are both useful techniques for diagnosing DON. Both CT scan and MRI can show apical crowding, muscle enlargement, intracranial fat prolapse, bony orbital angles that are correlated with occurrence of DON (Fig 1).
CT scans are chosen for surgical planning as it is readily accessible, fast to perform, less expensive and define bone better than MRI. Apical effacement of the optic nerve by more than 50% is both sensitive (80%) and specific (70.6%) in diagnosis of DON.[3][10][16] CT scans can also demonstrate changes to the angle of the inferomedial orbital strut (AIOS) and reduction of the angle of the medial wall (AMW),[25]orbital volume and size of globe. However, the role of orbital geometry, size of globe and orbital volume of bony orbit especially among different types of orbital geometry and races have not been clearly elucidated.[26] The DICOM data can also be used to plan surgery and for intraoperative guidance and navigational surgery. Steep bony orbital angles and intracranial orbital fat prolapse, seen in about 20% of cases, although non-specific, may be correlated with DON.[3][27]
MRI can provide additional findings regarding activity of the disease especially with STIR sequences.[28] An enlarged medial rectus or superior rectus/levator muscle complex increases the incidence of DON, apparently because of their proximity to the optic nerve as it passes between the annulus of Zinn.[29] [30]
Fat prolapse through the superior orbital fissure can be seen in 20% of patients [3]although not limited to patients with DON.
Figure 1. CT scan. orbital apex crowding. prominent enlargement of the four recti muscles complex on both sides in a 61 year old woman. Visual acuity 2 mCF and 20/200 in right and left eye, respectively.
Doppler sonography
This technique may show decreased or reversed flow of the superior orbital vein in compressive cases. Steep bony orbital angles and intracranial orbital fat prolapse are highly correlated with DON.[27]
Laboratory Tests
In one study it was found that 96% of patients with recent DON have high titers of thyroid-stimulating immunoglobulins (TSI). Serum TSI levels may help to identify patients with DON of recent onset.[31]TSI has positive correlation with disease activity in patients with TED. Further studies are needed to evaluate the possible role of serology tests to discriminate patients with DON among those with active without DON.
Management
Non-surgical approach
Medications
Corticosteroids
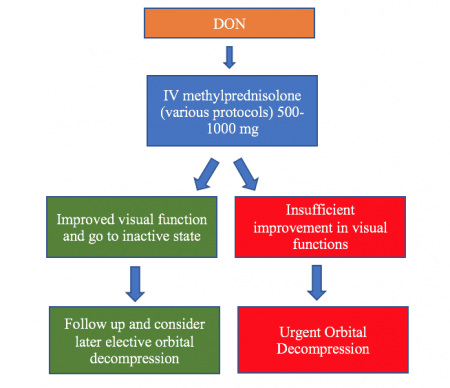
Intravenous corticosteroids (IVCS) are the first therapy in active TED and for cases of progressive compressive DON.[1]Intravenous methylprednisolone (IVMP) pulse therapy has greater efficacy and less adverse events compare to oral prednisolone. More severe complications are associated with doses exceeding 8 g per cycle and when administered daily.[16][32] EUGOGO suggested IVMP as the first line treatment in DON.[16]A variety of protocols have been suggested by different authors. One widely used regimen is the original EUGOGO protocol which suggested 500 mg IVMP every week for 6 weeks followed by 250 mg IVMP weekly for another 6 weeks. This protocol was proposed to improve the safety of IVMP administration as apposed to large daily disease of medication. Another regimen is 500mg-1000mg IVMP daily for 3 successive days to be repeated in one week if incomplete response occurs. The EUGOGO 2021 protocol advocates higher doses of unto 1gm daily for 3 days and repeated as necessary weekly if improvement is noticed, not exceeding 8gm. Early orbital decompression should be considered if insufficient or no improvement in optic nerve function is achieved within a few weeks following medical treatment. Complete visual recovery may occur in about 40% of patients with DON after treatment but far more so with surgical decompression.[16]
Immunosuppressive and Immunomodulatory agents
A variety of immunosuppressive and immunomodulating agents have been studied in patients with active TED and DON. These include Rituximab (an anti-CD20 monoclonal antibody) [33], Tocilizumab (an anti-IL-6 monoclonal antibody)[34]and more recently Teprotumumab (an anti-IGF-1R monoclonal antibody).[35] [36] [37]
Both rituximab and tocilizumab may be considered in patients with refractive active inflammatory orbitopathy but with some proven benefit in DON [3] [16] [38][39]Teprotumumab has proven effective in active inflammatory TED in reducing proptosis, and is now promising on treating chronic TED.
External beam radiotherapy (EBRT)
EBRT as adjunct to systemic corticosteroid is considered an alternate treatment of DON. It decreases inflammation by targeting lymphocytes and fibrocytes and may help in early progressive TED.[40]Radiotherapy takes longer to reduce inflammation in active TED compared to CS but has a synergistic affect with CS.[40] In a retrospective study, a large cohort of patients with active TED who received either CS alone or CS combined with EBRT were assessed for subsequent onset of DON: no patient with combined CS/EBRT developed DON compared with 17% of those in the CS group.[41]
Orbital Decompression
Surgical expansion of the orbital apex can provide prompt improvement of DON and remains the gold standard in severe and refractory cases. The goal is to achieve apical orbital volume expansion – both along the medial wall, the orbital floor including the palatine process and possibly the deep lateral wall. Urgent orbital decompression is considered if there is no improvement of optic nerve function within 2 weeks following medical treatment. In contrast to elective surgery in other non-sight threatening conditions, surgery is often offered during the progressive phase. [10] Several studies have shown that different methods of decompression including medial wall, the inferior wall, deep lateral wall, and the orbital fat, can all be effective in patients with DON. Bilateral orbital decompression may be performed concurrently or sequentially. Approaches to the orbit may include transcutaneous approach (the upper eyelid crease approach to the deep lateral wall), medial and inferior transconjunctival approaches (to the medial wall and floor including the posterior strut) and trans nasal endoscopic approaches have also been found to be effective. Preoperative treatment planning and intraoperative navigation is often helpful to minimize under decompression , avoid complications including CSF leaks. Sometime, postoperative immunosuppression for persistent active inflammatory disease may have to be continued until quiescence.
Even if vision loss has been chronic and severe, orbital decompression can save vision in some patients. In a cases series by Devoto et al, a 43yo patient with progressive vision loss of CF right and NLP left eyes and after decompression via medial wall (transcaruncular) and posterior medial floor, the vision recovered to 20/20 bilateral, while another 68yo patient with 3month vision loss to CF right and NLP left eye recovered to 20/400 and 20/50 respectively after decompression. <[42]
A Word of Caution-steroids prior to RAI
Radioactive iodine (RAI) is a well known treatment for persistent or refractory hyperthyroidism. However, it has been reported that even up to 20% of patients with preexisting TED can worsen symptoms, while the risk of developing severe TED is ~7%[43], and vision can worsen, [44] even to the point of compressing the optic nerve further. It is hypothesized that RAI releases thyroid antigens that are then directed toward the orbit.
In few case reports If the patients were treated with prednisone prior to radiation, the progression of ophthalmopathy was not seen[45]. Therefore, as a general recommendation, the patients with TED who are scheduled to receive RAI, are placed on peri-RAI steroids. This is particularly important in patients that have pre-existing TED with enlarged EOM that are very close to the optic nerve.
Factors associated to worsening TED after RAI
- Smoking
- High triiodothyronine in serum pre-RAI treatment
- Hypothyroidism post-RAI treatment
Therefore, optimization of the above may help to reduce risk of worsening TED after RAI.
Summary
In summary, DON is an infrequent yet devastating consequence of Thyroid Eye Disease. Prompt recognition, confirmation with appropriate investigations, institution of medical followed by surgical treatment with postoperative rehabilitation help restore vision and and patient satisfaction.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 McKeag D, Lane CM, Lazarus JH et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy survey. Br J Ophthalmol 2007;91:455–458.
- ↑ 2.0 2.1 2.2 2.3 Dolman PJ. Evaluating graves orbitopathy. Best Pract Res Clin Endocrinol Metab 2012;26(3):229–248.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 Saeed P, Tavakoli Rad S, Bisschop PHLT. Dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg 2018;34(4 Suppl 1):S60–S67.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Blandford AD, Zhang D, Chundury RV, Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Rev Ophthalmol 2017;12(2):111–121.
- ↑ 5.0 5.1 Bahn RS. Current insights into the pathogenesis of Graves’ ophthalmopathy. Horm Metab Res 2015;47(10):773–778.
- ↑ Smith TJ. Teprotumumab in thyroid-associated ophthalmopathy: rationale for therapeutic insulin-like growth factor-I receptor inhibition. J Neuroophthalmol 2020;40(1):74–78.
- ↑ Dolman PJ. Grading severity and activity in thyroid eye disease. Ophthal Plast Reconstr Surg 2018;34(4S):S34–S40.
- ↑ Abraham-Nordling M, Byström K, Törring O, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol 2011;165:899–905.
- ↑ De Silva C, et al. Risk factors for Graves’ Orbitopathy; the Australian Thyroid-Associated Orbitopathy Research (ATOR) study. J Clin Endocrinol Metab 2016;101:2711–20.
- ↑ 10.0 10.1 10.2 10.3 10.4 Dolman PJ. Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Invest, 2020;1-9.
- ↑ 11.0 11.1 Khong JJ, Finch S, De Silva C et al. Risk factors for Graves’ orbitopathy; the Australian Thyroid-Associated Orbitopathy Research (ATOR) Study. J Clin Endocrinol Metab 2016;1010(7):2711–2720.
- ↑ Kalmann R, Mourits MP. Diabetes mellitus: a risk factor in patients with Graves’ orbitopathy. Br J Ophthalmol 1999;83(4):463–465.
- ↑ 13.0 13.1 Day RM, Carroll FD. Corticosteroids in the treatment of optic nerve involvement associated with thyroid dysfunction. Trans Am Ophth Soc 1967; 65:41–51.
- ↑ Kazim M, Trokel SL, Acaroglu G, Elliott A. Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol 2000;84(6):600–605.
- ↑ Rose GE, Vahdani K. Optic nerve stretch is unlikely to be a significant causative factor in dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg 2020;36(2):157–163.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 16.8 16.9 Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM; European Group on Graves' Orbitopathy (EUGOGO). The 2016 European Thyroid Association/European Group on Graves' Orbitopathy. Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J. 2016;5(1):9-26.
- ↑ 17.0 17.1 17.2 Wong Y, Dickinson J, Perros P et al. A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom. Eye 2018;32(10):1555–1562
- ↑ 18.0 18.1 Dolman PJ, "Predictors of disease severity in thyroid-related orbitopathy.," in Orbital Disease. Present status and future challenges. Taylor and Francis, 2005, (chap18).
- ↑ Wiersinga WM, "Graves' Orbitopathy: A Multidisciplinary Approach - Questions and Answers.," Basel, Karger 2017;193-201.
- ↑ 20.0 20.1 Rootman JR, "Dysthyroid optic Neuropathy," in Diseases of the Orbit: A Multidisciplinary Approach. 2nd ed., Philadelphia, PA: Lippincott Williams & Wilkins, 2003.
- ↑ 21.0 21.1 Choi CJ, Oropesa S, Callahan AB, Glass LR et al. Patterns of visual field changes in thyroid eye disease. Orbit 2017;36(4):201–207.
- ↑ Birchall D, Goodall KL, Noble JL, Jackson A. Graves ophthalmopathy: intracranial fat prolapse on CT images as an indicator of optic nerve compression. Radiology. 1996;200(1):123-7.
- ↑ Lao TW, Rong SS, Ling AN, Brelén ME et al. Electrophysiological studies in thyroid associated orbitopathy: a systematic review. Curr Opin Neurol 2019;32(1):115–123.
- ↑ Micieli JA, Newman NJ, Biousse V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Expert Rev Ophthalmol 2017;12(2):111–121.
- ↑ Tan NYQ, Leong YY, Lang SS, Htoon ZM, Young SM, Sundar G. Radiologic Parameters of Orbital Bone Remodeling in Thyroid Eye Disease. Invest Ophthalmol Vis Sci. 2017 May 1;58(5):2527-2533.
- ↑ Weis E, "Quantitative computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: a volumetric analysis.," Ophthalmology 2021;119(10): 2174-8, 2021.
- ↑ 27.0 27.1 Chan LL, Tan HE, Fook-Chong S, Teo TH, Lim LH, Seah LL. Graves ophthalmopathy: the bony orbit in optic neuropathy, its apical angular capacity, and impact on prediction of risk. AJNR Am J Neuroradiol. 2009; 30(3):597-602.
- ↑ Dodds NI, Atcha AW, Birchall D, Jackson A. Use of high-resolution MRI of the optic nerve in Graves' ophthalmopathy. Br J Radiol. 2009;82(979):541-4.
- ↑ Giaconi JA, Kazim M, Rho T et al. CT scan evidence of dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg 2002;18(3):177–182.
- ↑ Starks VS, Reinshagen KL, Lee NG, Freitag SK. Visual field and orbital computed tomography correlation in dysthyroid optic neuropathy due to thyroid eye disease. Orbit. 2020;39(2):77-83.
- ↑ Ponto KA, Diana T, Binder H, Matheis N, Pitz S, Pfeiffer N, Kahaly GJ. Thyroid-stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. J Endocrinol Invest. 2015;38(7):769-77.
- ↑ Currò N, Covelli D, Vannucchi G, Campi I, Pirola G, Simonetta S, Dazzi D, Guastella C, Pignataro L, Beck-Peccoz P, Ratiglia R, Salvi M. Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014;24(5):897-905.
- ↑ Salvi M, Vannucchi G, Curro N et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 2015;100:402–431.
- ↑ Perez-Moreiras JV et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: a randomized clinical trial. Am J Ophthalmol 2018;195:181–190.
- ↑ Douglas RS, Kahaly GJ, Patel A, Sile S et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med 2020;382:341–352.
- ↑ Sears CM, Azad AD, Dosiou C, Kossler AL. Teprotumumab for Dysthyroid Optic Neuropathy: Early Response to Therapy. Ophthalmic Plast Reconstr Surg. 2020; 22
- ↑ Hwang CJ, Nichols EE, Chon BH, Perry JD. Bilateral dysthyroid compressive optic neuropathy responsive to teprotumumab. Eur J Ophthalmol. 2021;1:1120672121991042.
- ↑ Slentz DH, Smith TJ, Kim DS, Joseph SS. Teprotumumab for Optic Neuropathy in Thyroid Eye Disease. JAMA Ophthalmol. 2021;139(2):244-247. doi:10.1001/jamaophthalmol.2020.5296
- ↑ Hwang CJ, Nichols EE, Chon BH, Perry JD. Bilateral dysthyroid compressive optic neuropathy responsive to teprotumumab. Eur J Ophthalmol. 2022;32(3):NP46-NP49. doi:10.1177/1120672121991042
- ↑ 40.0 40.1 Dolman PJ, Rath S. Orbital radiotherapy for thyroid eye disease. Curr Opin Ophthalmol 2012;23(5):427–432
- ↑ Shams PN, Ma R, Pickles T, Rootman J, Dolman PJ. Reduced risk of compressive optic neuropathy using orbital radiotherapy in patients with active thyroid eye disease. Am J Ophthalmol. 2014 Jun;157(6):1299-305
- ↑ >Devoto MH, Golnik K, Bernardini FP, Alencar VM. Improvement from no light perception after orbital decompression for graves' optic neuropathy. Ophthalmology. 2014 Jan;121(1):431-432.e1. doi: 10.1016/j.ophtha.2013.09.031. Epub 2013 Nov 20. PMID: 24268859.
- ↑ Ponto KA, Zang S, Kahaly GJ. The tale of radioiodine and Graves' orbitopathy. Thyroid. 2010;20(7):785-793. doi:10.1089/thy.2010.1640
- ↑ Salleh NA, Seng WH, Isa HD. Optic Neuropathy in Thyroid Eye Disease: A Case Series. Korean J Fam Med. 2016 May;37(3):197-201. doi: 10.4082/kjfm.2016.37.3.197. Epub 2016 May 26. PMID: 27274392; PMCID: PMC4891323.
- ↑ Walsh JP, Dayan CM, Potts MJ. Radioiodine and thyroid eye disease. BMJ. 1999;319(7202):68-69. doi:10.1136/bmj.319.7202.68