Dupilumab-Induced Conjunctivitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Dupilumab (Dupixent® by Regeneron & Sanofi, USA), is the first FDA-approved biologic systemic treatment for atopic dermatitis. This human monoclonal antibody binds to interleukin-4 receptor subunit alpha (IL-4Ra) and effectively blocks IL-4 and IL-13 signaling, which induces the inflammatory process responsible for asthma, atopic dermatitis, and chronic sinusitis with nasal polyps.[1][2] In Phase 3 of clinical trials, the incidence of conjunctivitis (including viral, bacterial, allergic conjunctivitis, and atopic keratoconjunctivitis) was higher in patients treated with dupilumab and topical corticosteroids compared to the control group treated with placebo and topical corticosteroids (8% vs 14-19%). Most cases of conjunctivitis in clinical trials were reportedly mild, and only one patient discontinued dupilumab due to severe unilateral atopic keratoconjunctivitis.[1] However, since its FDA approval in March 2017, there has been an increasing number of reports of severe dupilumab-induced conjunctivitis, which warrants a closer look at this disease entity.
Risk Factors
Dupilumab dosage, the severity of atopic dermatitis, and the presence of conjunctivitis before dupilumab treatment were found to be independent risk factors for dupilumab-induced conjunctivitis. [reference needed]
Pathology
Conjunctival biopsies of patients with ophthalmologist-confirmed conjunctivitis during dupilumab treatment revealed a scarcity of intraepithelial goblet cells with a median density of 3.3 cells/mm vs. 32.3 cells/mm in control groups. CD3+/CD4+ T cells and eosinophils were also found migrating into the conjunctival epithelium. Interestingly, this is different from histopathological findings of atopic keratoconjunctivitis and allergic conjunctivitis, in which there is an increased density of goblet cells.[3]
Pathophysiology
There have been a few proposed mechanisms to explain the development of conjunctivitis in patients with atopic dermatitis treated with dupilumab.
- Thyssen theorized that suppressing IL-4 and IL-13 signaling led to an increased number of Demodex, which in turn caused an IL-17-mediated rosacea-like inflammation.[4]
- Bakker et al. thought the decreased density of conjunctival goblet cells in the settings of increased lymphocytes, as well as eosinophils, plays a key role in the inflammatory process.[3]
- Akinlade et al, through pharmacokinetic data, suggested that the lower tissue distribution of dupilumab in the eyes might be related to more inflammation here compared to elsewhere.[5] This theory is consistent with the fact conjunctivitis is specific to atopic dermatitis treated with dupilumab, as this side effect has not been reported in other conditions treated with this drug.[6]
Diagnosis
Dupilumab-induced conjunctivitis ranges from mild to severe blepharitis, conjunctivitis, and keratoconjunctivitis. The diagnosis is made clinically based on history and ophthalmic examination.
History
Patients must have a history of moderate to severe atopic dermatitis (also known as eczema) recalcitrant to topical therapy, and have recently started dupilumab treatment. There is frequently known ocular surface disease before starting dupilumab, as this is a well-known comorbidity of atopic disorders, including eczema, asthma, and allergic rhinitis.[7][8][9][10][11][12][13][14][15] Symptoms develop quickly in patients on dupilumab monotherapy (after about 2 weeks on average) vs. dupilumab + topical corticosteroids (after about 4-8 weeks).[5]
Symptoms
Patients typically present with these symptoms:
- Redness
- Irritation
- Itching
- Foreign body sensation
- Photophobia
- Tearing
- Discharge
- Decreased vision
- Intermittent monocular diplopia
Physical examination
Providers should pay attention to the anterior segment and note any sign of inflammation, which is usually bilateral but asymmetric.
1. Early stage:
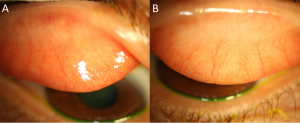
- Meibomian gland dysfunction
- Conjunctival hyperemia
- Papillary conjunctivitis (Figure 1)
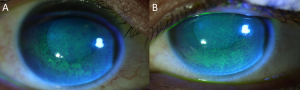
- Superficial punctate keratopathy (Figure 2)
2. Intermediate stage:
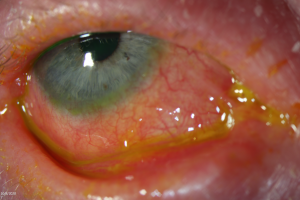
- Symblepharon (Figure 3)
- Madarosis
- Shortening of fornices
- Limbitis
3. Advanced stage:
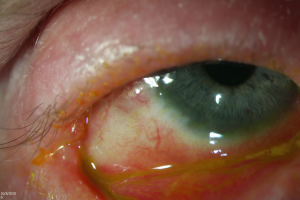
- Ankyloblepharon (Figure 4)
- Complete loss of fornices
- Ocular surface keratinization
In addition, a few special tests could be performed in-office to determine the stage and prognosis:
- Corneal sensation test using cotton tip applicator or Cochet-Bonnet esthesiometer
- Schirmer's test: Less than 10 mm of moisture may indicate the involvement of lacrimal glands in an advanced disease
- Ocular surface staining with fluorescein and lissamine green, which is useful to assess conjunctival integrity
- Tear break-up time (TBUT)
- Delayed tear clearance
In cases with risk factors such as pre-existing ocular surface disease or severe atopic dermatitis of eyelids, a baseline ophthalmic examination and frequent follow-up with an ophthalmologist after initiation of treatment may be useful. Close monitoring with treatment in the setting of active inflammation may be warranted, given the reversible nature of the early disease. Slit-lamp photographs should be considered at each visit to document baseline, progression/improvement, and response to treatment.
Laboratory test
Diagnosis is based on clinical history and examination. No laboratory test or biopsy is indicated.
Differential diagnosis
It is important to rule out other etiologies through history and physical examination to tailor the best course of treatment:
- Dry eye syndrome
- Chemical burn
- Scleritis
- Episcleritis
- Viral conjunctivitis
- Bacterial conjunctivitis
- Allergic conjunctivitis
- Atopic keratoconjunctivitis
- Ocular cicatricial pemphigoid
- Ocular graft-versus-host disease
- Ocular Stevens-Johnson syndrome/toxic epidermal necrolysis
Management
Medical treatment
The main goal of treatment is to halt the inflammatory process. First-line therapy includes topical steroids and/or topical immunomodulators such as tacrolimus or cyclosporine. However, treatment choice may not be straightforward, given the potential side effects of these medications.
Wallenberg et al. reported 11 cases of mild to severe dupilumab-induced conjunctivitis that showed excellent results with topical fluorometholone and tacrolimus, a calcineurin inhibitor. Among 5 patients treated with fluorometholone 0.1% drop, 2 patients had complete resolution of conjunctivitis, while 3 significantly improved. In 4 cases treated with tacrolimus 0.03% ointment, 2 patients had complete resolution, whereas the other 2 significantly improved in both signs and symptoms. The remaining patients were treated with either dexamethasone, hydrocortisone, or an antibiotic and steroid combination topical medication, and all cases reported improvement. In another report, two cases were treated successfully with an initial dose of prednisolone acetate 1% eye drops with concurrent cyclosporine 0.05% eye drops, a calcineurin inhibitor, for maintenance without recurrence of conjunctivitis despite the continuation of dupilumab injections.[16] It is postulated that if a lower number of goblet cells play a meaningful role in the mechanism of the disease process, calcineurin inhibitors such as cyclosporine may reverse this process by increasing goblet cells while preventing epithelial cell death.[17]
Since other therapies such as topical antihistamines and artificial tears were ineffective, lower dose steroids such as fluorometholone and/or a non-steroid immunomodulatory agent such as tacrolimus and cyclosporine should be initially considered to prevent the adverse effects of topical steroids such as the development of cataract or glaucoma.[16]
Surgical treatment
There are no reports of surgical treatment for dupilumab-induced conjunctivitis.
Prognosis
Nearly all cases of dupilumab-induced conjunctivitis and ocular surface disease resolved with the initiation of medical treatment and/or discontinuation of dupilumab. However, discontinuation is usually not needed.
References
- ↑ 1.0 1.1 Blauvelt A, Bruin-Weller M, Gooderham M, et al., Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303.
- ↑ Gandhi N, Bennett B, Graham N, Pirozzi G, Stahl, Yancopoulos G. Targeting key proximal drivers of type 2 inflammation in disease. Nature Reviews Drug Discovery. 2016;15(1):35-50.
- ↑ 3.0 3.1 Bakker DS, Ariens LFM, van Luijk C, van der Schaft J, Thijs JL, Schuttelaar MLA, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180(5):1248-9.
- ↑ Thyssen JP. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin‐17 levels? Br J Dermatol 2018; 178: 1220.
- ↑ 5.0 5.1 Akinlade B, Guttman-Yassky E, de Bruin-Weller M, Simpson EL, Blauvelt A, Cork MJ, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181(3):459-73.
- ↑ Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, Chao J, Staudinger H, Pirozzi G, Antoni C, Amin N, Ruddy M, Akinlade B, Graham NMH, Stahl N, Yancopoulos GD, Teper A. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med. 2018 Jun 28; 378(26):2475-2485.
- ↑ Bousquet J, Devillier P, Anto JM et al. Daily allergic multimorbidity in rhinitis using mobile technology: a novel concept of the MASK study. Allergy 2018; 73: 1622– 31.
- ↑ Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol 2011; 11: 471– 6.
- ↑ Maio S, Baldacci S, Bresciani M et al. RItA: the Italian severe/uncontrolled asthma registry. Allergy 2018; 73: 683– 95.
- ↑ Lemmetyinen RE, Karjalainen JV, But A et al. Higher mortality of adults with asthma: a 15‐year follow‐up of a population‐based cohort. Allergy 2018; 73: 1479– 88.
- ↑ Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol 2014; 98: 1712– 17.
- ↑ Kim M, Oh J‐H, Park CY, Lee SW. Dry eye disease and allergic conditions: a Korean nationwide population‐based study. Am J Rhinol Allergy 2016; 30: 397– 401.
- ↑ Cingi C, Gevaert P, Mösges R et al. Multi‐morbidities of allergic rhinitis in adults: European Academy of Allergy and Clinical Immunology Task Force Report. Clin Transl Allergy 2017; 7: 17.
- ↑ Michailopoulos P, Almaliotis D, Georgiadou I et al. Allergic conjunctivitis in patients with respiratory allergic symptoms; a retrospective study in Greece. Med Hypothesis Discov Innov Ophthalmol 2017; 6: 3– 9.
- ↑ Wang X, Shi X‐D, Li L‐F et al. Prevalence and clinical features of adult atopic dermatitis in tertiary hospitals of China. Medicine (Baltimore) 2017; 96: e6317.
- ↑ 16.0 16.1 Wollenberg A, Ariens L, Thurau S, van Luijk C, Seegräber M, de Bruin-Weller M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;5–8.
- ↑ Maudinet A, Law-Koune S, Duretz C, Lasek A, Modiano P, Tran THC. Ocular Surface Diseases Induced by Dupilumab in Severe Atopic Dermatitis. Ophthalmol Ther. 2019;8(3):485-490.