Drug-induced Acute Angle Closure Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Epidemiology and Risk factors
Glaucoma is a form of optic neuropathy. It is usually associated with intra-ocular pressure (IOP) abnormalities, thinning of the retinal nerve fiber layer (RNFL), characteristic visual field loss, and may be categorized as open or closed angle based on the status of the iridocorneal angle.
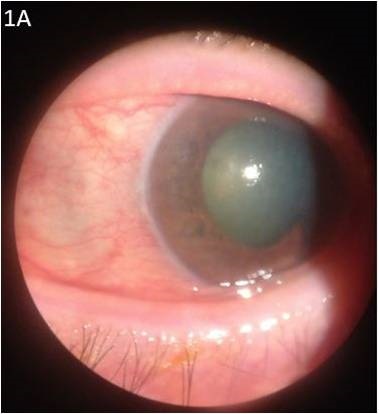
Several topical and systemic medications may induce acute angle closure (AAC) resulting in acute IOP elevation. Patients predisposed to AAC are classically farsighted (hypermetropic), with a shorter axial length, narrow angles (3.8% prevalence in Whites and 8.5% in Asians), and shallow anterior chambers. Drug-induced AAC is an ophthalmic emergency which may lead to persistent visual loss from acute angle closure glaucoma (AACG) if not treated emergently. Presenting symptoms include conjunctival hyperemia, nausea, emesis, acute onset of impaired vision, ocular, periocular pain, colored halos and headache.
Mechanism
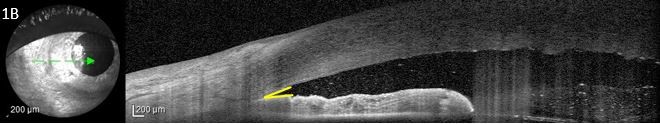
- Pupillary block AACG: Drugs with sympathomimetic or parasympatholytic properties can cause pupillary block in susceptible patients with narrow iridocorneal angles. In pupillary block AACG, 360 degree contact is formed between the pupillary margins and the lens, blocking flow of aqueous humor from the posterior chamber to the anterior chamber through the pupil, resulting in a pressure gradient between the two chambers. The elevated pressure in the posterior chamber exacerbates the bowing of the lens-iris diaphragm, leading to narrowing of the irido-corneal angle and to increased IOP.
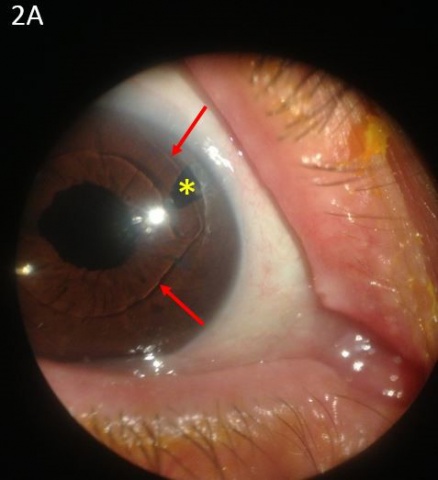
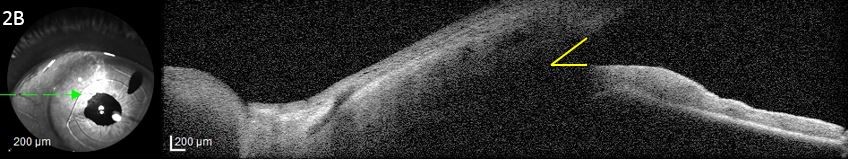
- Non-pupillary block AACG: Thickening and forward movement of lens-iris diaphragm, forward rotation of the ciliary body, and choroidal effusion may occur in patients with either open or narrow angles leading to AACG. This process appears to be an idiosyncratic drug reaction to certain systemic medications.
Ocular agents
- Topical cholinergic or anticholinesterase agents can induce AACG due to anterior movement of the lens-iris diaphragm[1]
- Sympathomimetics, especially those with Alpha-1 agonistic activity, cause mydriasis and can precipitate AACG in predisposed individuals. Topical phenylephrine, used in-office for iris dilation, and its prodrugs dipivefrin and apraclonidine have been documented to induce AACG [2]
- Topical anticholinergic/cycloplegics agents used for pupillary dilation may also lead to AACG [2][1]
- Botulinum act at peripheral cholinergic synapses, inhibiting the release of acetylcholine. When injected periocularly (e.g., for hemifacial spasm), Botulinum can cause pupillary dilation and can induce AACG [3]
Antibacterial agents
- Sulfa drugs may induce AACG without pupillary block. Mechanisms in this type of angle closure include lenticular swelling, retinal edema and choroidal effusion resulting in secondary shallowing of the anterior chamber [4]
- Gentamycin has also been reported to induce mydriasis after topical use in a patient, which theoretically can cause AACG in individuals with narrow iridocorneal angles [5]
CNS agents
- Antidepressants may lead to AACG due to their anticholinergic effects which cause pupillary dilatation, and increased aqueous production secondary to increased ciliary body blood flow. Both tricyclic agents (amitriptyline and imipramine) and non-tricyclic drugs (mianserin hydrochloride, paroxetine, fluoxetine, maprotiline, fluvoxamine, venlafaxine, citalopram, and escitalopram) have documented associations with AACG [6]
- Antipsychotics have a relatively weaker anticholinergic action and lower possibility of inducing AACG. Among the available antipsychotic agents, perphenazine, trifluperazine, and fluphenazine have been reported to induce AACG [6]
- Benzodiazepines can induce AACG, because they induce relaxation of the sphincter muscle of the iris and have a mild anticholinergic effect. Diazepam, clotiazepam and alprazolam have documented associations with AACG [6]
- Anti-Parkinsonians like cabergoline, a dopamine D2 receptor agonist, may induce nonpupillary block AACG associated with choroidal effusion [7]
- Anticonvulsant agent topiramate, a sulfamate-substituted monosaccharide antiseizure medication, is also used in the management of migraine, depression, idiopathic intracranial hypertension, and neuropathic pain. Topiramate can induce AACG within the first 2 weeks after initiation, and in almost all cases present in both eyes [8]
- Ecstasy, a synthetic amphetamine derivate, and marijuana have both been reported to induce recurrent bilateral AACG [9]. Cocaine has indirect sympathomimetic activity and causes mydriasis. AACG has been reported following therapeutic or abuse intranasal application of cocaine [10]
Respiratory agents
- Epinephrine may cause mydriasis and precipitate AACG in susceptible individuals [6]
- Ipratropium bromide, an anticholinergic agent, causes mydriasis and may precipitate AAC. Tiotropium bromide, another anticholinergic agent, has weaker anticholinergic activity, but has also been reported to induce AAC [6]
Cardiac agents
Hematologic agents
- Anticoagulants, by precipitating spontaneous choroidal hemorrhages, may also induce AACG [13]
Anti-inflammatory agents
- Promethazine, an H1-blocker agent, has been shown to produce an idiopathic swelling of the lens that can induce AACG [14]
- Mefenamic acid, a non-steroidal anti-inflammatory agent may induce secondary non-pupillary block AACG [15]
Gastrointestinal agents
- Cimetidine and ranitidine, H2-blocker agents, have weak anticholinergic adverse effects, which may induce AACG [16]
Management and prevention
Drug-induced AACG can be prevented in susceptible individuals through proper counselling of the medication risks associated with narrow angles and when appropriate, the placement of a prophylactic peripheral iridotomy to prevent acute angle closure. Because non-pupillary block causes of AACG may not respond to prophylactic iridotomies, a high degree of suspicion is necessary to identify these forms of drug-induced AACG. A B-scan or UBM can be helpful to identify choroidal or ciliary body expansion and rotation, and both topical and systemic IOP-lowering medications are the mainstays of treatment. Documentation and review of both topical and systemic medications, lens status, historical angle and iris anatomy, and laterality of acute angle closure can help to elucidate the cause in some cases. In the acute setting, it may be difficult to definitively differentiate between pupillary block and non-pupillary block mechanisms of AACG. In these cases, the placement of a laser iridotomy is the preferred initial step to both alleviate the pupillary block component of angle closure and to decrease IOP by creating an alternative pathway for aqueous to flow into the anterior chamber. Once the offending medication has been identified, a multi-specialty approach may be required to ensure a patient’s medications are appropriately adjusted.
References
- ↑ Jump up to: 1.0 1.1 Brooks, Anne MV, Robert H. West, and William E. Gillies. "The risks of precipitating acute angle‐closure glaucoma with the clinical use of mydriatic agents." (1986): 34-36.
- ↑ Jump up to: 2.0 2.1 Wolfs, R. C., et al. "Risk of acute angle-closure glaucoma after diagnostic mydriasis in nonselected subjects: the Rotterdam Study." Investigative ophthalmology & visual science 38.12 (1997): 2683-2687.
- ↑ Corridan, P., et al. "Acute angle-closure glaucoma following botulinum toxin injection for blepharospasm." British journal of ophthalmology 74.5 (1990): 309-310.
- ↑ Panday, Vasudha A., and Douglas J. Rhee. "Review of sulfonamide-induced acute myopia and acute bilateral angle-closure glaucoma." Comprehensive Ophthalmology Update 8.5 (2007): 271-276.
- ↑ Awan, Khalid J. "Mydriasis and conjunctival paresthesia from local gentamicin." American journal of ophthalmology 99.6 (1985): 723-724.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 Razeghinejad, M R et al. “Non-steroidal drug-induced glaucoma.” Eye (London, England) vol. 25,8 (2011): 971-80. doi:10.1038/eye.2011.128
- ↑ Razmjoo, Hasan, et al. "Bilateral angle-closure glaucoma in a young female receiving cabergoline: a case report." Case reports in ophthalmology 2.1 (2011): 30-33.
- ↑ Lee, Grace C., et al. "Bilateral angle closure glaucoma induced by sulphonamide‐derived medications." Clinical & experimental ophthalmology 35.1 (2007): 55-58.
- ↑ Trittibach, Peter, Beatrice E. Frueh, and David Goldblum. "Bilateral angle-closure glaucoma after combined consumption of" ecstasy" and marijuana." The American journal of emergency medicine 23.6 (2005): 813-814.
- ↑ Wilcsek, G A et al. “Acute angle closure glaucoma following the use of intranasal cocaine during dacryocystorhinostomy.” The British journal of ophthalmology vol. 86,11 (2002): 1312. doi:10.1136/bjo.86.11.1312
- ↑ Ahmad, Saeed. "Disopyramide: pulmonary complications and glaucoma." Mayo Clinic Proceedings. Vol. 65. No. 7. Elsevier, 1990.
- ↑ Trope, G. E., and V. M. D. Hind. "Closed-angle glaucoma in patient on disopyramide." The Lancet 311.8059 (1978): 329.
- ↑ Ah-Kee, Elliott Yann et al. “A review of drug-induced acute angle closure glaucoma for non-ophthalmologists.” Qatar medical journal vol. 2015,1 6. 10 May. 2015, doi:10.5339/qmj.2015.6
- ↑ Bard, Leslie A. "Transient myopia associated with promethazine (phenegan) therapy: report of a case." American journal of ophthalmology 58 (1964): 682-686.
- ↑ Vishwakarma, Parag, Ganesh V. Raman, and P. Sathyan. "Mefenamic acid-induced bilateral transient myopia, secondary angle closure glaucoma and choroidal detachment." Indian Journal of Ophthalmology 57.5 (2009): 398.
- ↑ Dobrilla, G., et al. "Exacerbation of glaucoma associated with both cimetidine and ranitidine." The Lancet 319.8280 (1982): 1078.