Diffuse Unilateral Subacute Neuroretinitis (DUSN)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Diffuse Unilateral Subacute Neuroretinitis (DUSN)
Authors
Kristen Ann Mendoza, MD; Stephanie Cramer, MD; Elizabeth Verner-Cole, MD; Andreas Lauer, MD
Disease
Diffuse unilateral subacute neuroretinitis (DUSN) is a multifocal chorioretinitis caused by a nematode.
Etiology
Several nematodes have been found to be etiologic agents of DUSN with Toxocara canis being the first to be described. Others include Baylisacaris procyonis (a parasite of raccoons and skunks, often found in the midwestern United States, it is a large nematode 1500 to 2000 μm), Ancylostoma caninum (a dog hookworm, found in the southeastern United States and Brazil, it is a small nematode 650 μm, or 400 -1000 μm),[1] Gnathostoma spinigerum (found in raw or undercooked fish and meat in India), Strongyloides stercoralis, and Brugia malayi. [2] [3] [4] [5] The trematode Alaria has been involved as well (found in undercooked frogs in Asian countries).[6]
Risk Factors
Predisposing factors include travel to or residence in the United States (particularly the midwest and southeast), Canada, the Caribbean, Brazil, Venezuela, Senegal, India, China, Malaysia, Spain, Germany, and the United Kingdom, where causative parasites reside.[2][7] [8] DUSN occurs more frequently in children and young adults prior to the fourth decade, more often in males than females.
General Pathology
The nematode eggs are ingested after they have been shed from carriers such as dogs or raccoons. The nematode may also invade cutaneously. The nematode migrates hematogenously over the course of months and resides in the fundus for up to three years.[2][7][8][9] Infection results in an insidious, severe unilateral loss of peripheral and central vision due to retinal inflammation and degeneration. The retina, subretinal space, optic nerve, and choroid have been shown to manifest changes in DUSN.
Pathophysiology
The nematode and its waste products are thought to induce damage through toxic, inflammatory, and autoimmune mechanisms. It has been suggested that the outer retinal layer experiences a local toxic effect, whereas the inner retinal layer experiences a diffuse toxic reaction. Nematodes reside most often in the subretinal space, but they have also been located intraretinally. Lesions can disappear and then reappear in other areas of the retina, indicating the migratory path of the worm. Classically, DUSN occurs unilaterally, though, bilateral cases have been reported. [6][10] [11]
Primary prevention
Prevention includes avoidance of endemic areas and carrier animals of etiologic worms.
Diagnosis
DUSN is classified in early and late stages, based on clinical features. Definitive diagnosis is made with the rare visualization of the nematode. Although the worm can be identified at any stage, there may be a greater likelihood of locating the nematode in the early stages of the disease. Typically the worms are very mobile and rapidly shift location.
History
Patients may report travel to humid and warm endemic areas, and/or exposure to animal carriers.
Physical examination
Visual acuity on initial presentation varies, and has been found to be 20/20[12] or worse than 20/400.[11]
Signs
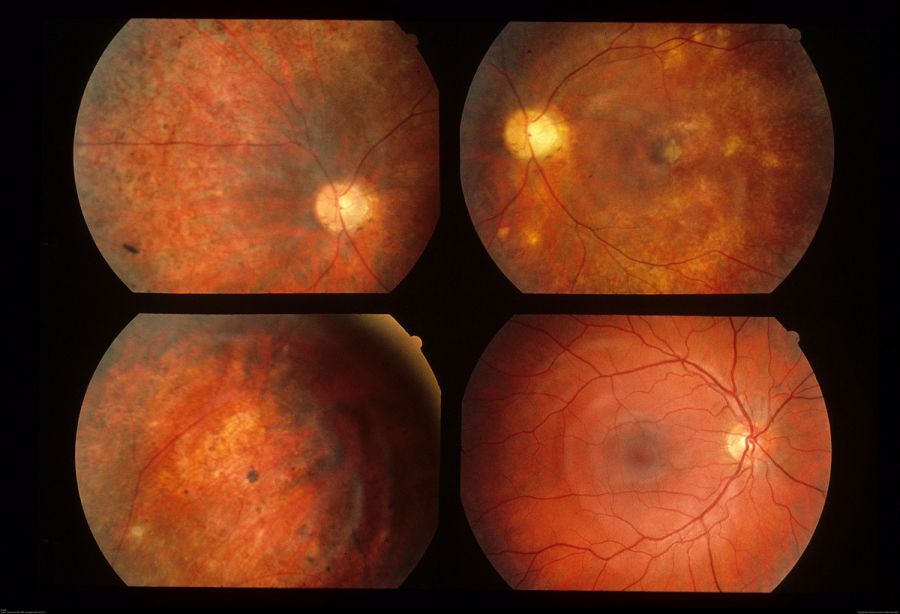
Early Stage: mild optic nerve edema, mild to moderate vitritis, papillitis, and clustered yellow-gray-white lesions
Late Stage: optic nerve atrophy, retinal arteriolar narrowing, increased internal limiting membrane reflex (Oréfice’s sign), subretinal tunnels (Garcia’s sign), diffuse retinal pigment epithelium (RPE) degeneration, and afferent pupillary defect
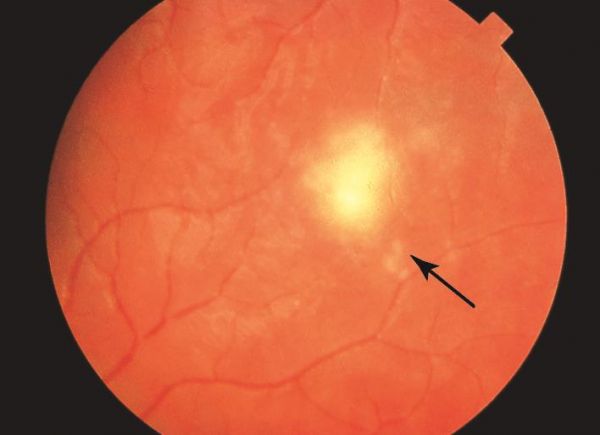
Overall, the most common features seen in early and late DUSN are subretinal tracks (91.7%), focal RPE changes (89.3%), and small white subretinal spots (80.2%). When the worm is able to be localized, it is most often in the posterior pole (43%), followed by the temporal retina (27%).[13] If the worm is visualized, it is often motile, slowly coiling and uncoiling. Depending on the species, the worm is 400 to 2000 micrometers in length, tapered at both ends, and glistening white in color.[11]
Symptoms
Symptoms of DUSN include central or paracentral scotomas, acute (early stage) or progressively severe (late stage) decreased vision, visual field deficits, and new floaters. Ocular pain is not common.
Clinical diagnosis
DUSN is largely a clinical diagnosis as a worm is only identified in 25%[8] to 39%[14] of patients.
A nice video is available at http://aao-resources-enformehosting.s3.amazonaws.com/resources/AAO.LMS/BCSC_Media/BCSC1920/videos/BCSC2019_s09-v1101_diffuse-unilateral.mp4
Diagnostic procedures
Fundus examination: If the nematode can be seen, it is most often found in the macula near yellow-gray-white retinochoroidal lesions. In addition, optic nerve edema or atrophy, vascular narrowing, RPE changes, and vitritis may be noted.[8]
Electroretinography: Although the a:b ratio can be normal in the early stages, b-wave depression becomes proportional to retinal involvement.[8] Presumably, the inner retina is more profoundly affected than the outer retina.[15]
Electro-oculography: Reduced amplitudes are proportional to the degree of retinal damage, and can even become undetectable,[16] however about half of electro-oculographic exams are normal.
Ocular coherence tomography (OCT): Degeneration of the retinal nerve fiber layer (RNFL) and thinning of the macula are proportional to the degree of vision loss.[14][17] Of note, the RNFL may show an increase in thickness due to initial transitory edema. The inner retinal volume is significantly more affected than the outer retinal volume.[15] Loss of foveal depression and increased intraretinal hyperreflectivity in the shape of the worm can be seen as well.[17]
Fluorescein angiography: Diffuse changes in the RPE, capillary dye leaks near the optic nerve, and increased background choroidal fluorescence have been observed.[11][18] Indocyanide green angiography: Hypofluorescent spots correspond to choroidal infiltration and inflammation.[6][17]
Scanning laser ophthalmoscopy: This modality can facilitate visualization of the nematode.
Laboratory test
Serum eosinophilia may raise suspicion for the presence of a nematode.
Differential diagnosis
Early Stage: optic neuritis, pars planitis, histoplasmosis, toxoplasmosis, syphilitic chorioretinitis, sarcoidosis, acute posterior multifocal placoid pigment epitheliopathy, and multiple evanescent white dot syndrome.
Late Stage: unilateral retinitis pigmentosa, occlusive vascular disease, toxic retinopathy, and posttraumatic chorioretinopathy
Management
Visualization of the worm is rare. When the nematode is seen, it tends to migrate away from light and from view. Therefore it is important to immediately treat a visible nematode with photocoagulation. When clinical features are present in the absence of a visible worm, pharmacologic management may be commenced.
General treatment
Localization of the worm and subsequent photocoagulation is preferred, as elimination of the nematode halts vision loss and results in regression of inflammation. When visualization of the worm is not possible, all of the prime signs of early DUSN, as discussed above, should be present before antihelminitic drugs are initiated. Additionally, antihelmintic therapy may be useful adjunctively when laser treatment alone does not kill the worm.[19] [20] Oral corticosteroids may help decrease inflammatory responses in the eye as well.
Medical therapy
Systemic therapy: Although thiabendazole has been used previously, albendazole dosed at 400 mg/day for 30 days has been studied more often and with successful results.[2][8] Although generally well tolerated, side effects of albendazole include gastrointestinal upset, dizziness, rash, and alopecia. Albendazole is not recommended for patients who are pregnant or breast-feeding due to its teratogenic effects.[21] Corticosteroid therapy: Prednisone 40-60 mg daily, followed by a taper over 2 to 4 weeks is acceptable.[20][21]
Medical follow up
Changes in the retinal architecture corresponding to visual function can be monitored with imaging modalities such as the OCT. Perimetry can also monitor visual field.
Surgery
Photocoagulation: The location of the worm is confirmed by high-magnification biomicroscopy and the use of fundus contact lenses. The worm is then confined by a laser boundary using contiguous 200-micrometer spots with a 532-nanometer laser at about 200 milliwatts (mW) with a duration of up to 0.2 seconds, followed by confluent treatment within the bounded area. Ideally, the burn is sufficiently intense so that shriveling of the worm is seen. Alternatively, a single laser shot aimed at the head of the worm may be used.[18] Since the nematode can be photophobic, for safer photocoagulation, the slit beam may be aimed adjacent to the worm to direct it away from important regions such as the macula.
Extraction: Direct aspiration using pars plana vitrectomy has also been described.[22] [23]
Surgical follow up
Routinely monitor success and complications of the surgery post-operatively.
Complications
Areas of direct helminthic damage prior to therapy are often not reversible.[8] However, secondary inflammation following photocoagulation usually subsides after without sequelae.[18][24] [25] [26] [27] [28] Photocoagulation may be complicated by hemorrhage, vascular occlusion, choroidal neovascularization and epiretinal membrane formation.
Prognosis
Early detection and treatment stops vision loss, and complete resolution may be observed. In later stages, treatment is indicated to prevent additional vision loss. Failure to eliminate the nematode results in progressive chorioretinal destruction and severe, irreversible vision loss.[8]Visual acuity in late-stage DUSN does not improve by medical or surgical treatments.
References
- ↑ Mazzeo, T.J.M.M., dos Santos Motta, M.M. & Curi, A.L.L. Diffuse unilateral subacute neuroretinitis: review article. J Ophthal Inflamm Infect 9, 23 (2019). https://doi.org/10.1186/s12348-019-0191-x
- ↑ 2.0 2.1 2.2 2.3 Oueghlani E, O’Sullivan E, Pavesio CE. Diffuse unilateral subacute neuroretinitis in the United Kingdom. Int. Ophthalmol. 2010;30(5):615-9.
- ↑ Gass JDM, Gilbert WR, Guerry RK, Scelfo R: Diffuse unilateral subacute neuroretinitis. Ophthalmology. 1978;85:521-45.
- ↑ Gass JDM. Stereostopic atlas of macular diseases: Diagnosis and Treatment. St. Louis: Mosby; 1987. 470-5.
- ↑ Kazacos KR, Raymond LA, Kazacos EA, Vestre WA. The raccoon ascarid: a probable cause of human ocular larva migrans. Ophthalmology. 1985;92:1735-44.
- ↑ 6.0 6.1 6.2 Garcia CA, Sabrosa NA, Gomes AB, Segundo Pde S, Garcia Filho CA, Sabrosa AS. Diffuse unilateral subacute neuroretinitis—DUSN. Int. Ophthalmol. Clin. 2008;48(3):119-29.
- ↑ 7.0 7.1 Yusoff M, Alwi AA, Said MM, Zakariah S, Ghani ZA, Zunaina E. Intraocular nematode with diffuse unilateral subacute neuroretinitis: case report. BMC Ophthalmol. 2011;11:15.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Roy FH, Fraunfelder FW, Fraunfelder FT. Current Ocular Therapy. 6th edition. Elsevier; 2008. 622-3.
- ↑ Gass JDM, Scelfo R. Diffuse unilateral subacute neuroretinitis. J. R. Soc. Med. 1978;71:95-111.
- ↑ de Souza EC, Abujamra S, Nakashima Y, Gass JD. Diffuse bilateral subacute neuroretinitis: first patient with documented nematodes in both eyes. Arch. Ophthalmol. 1999;117(10):1349-51.
- ↑ 11.0 11.1 11.2 11.3 Arevalo JF, Arevalo FA, Garcia RA, de Amorim Garcia Filho CA, de Amorim Garcia CA. Diffuse unilateral subacute neuroretinitis. J. Pediatr. Ophthalmol. Strabismus. 2013;(4):204-12.
- ↑ Goldberg N, Bhatnagar P. Letter to the Editor. Diffuse unilateral subacute neuroretinitis (DUSN). Ocul. Immunol. Inflamm. 2012;20(2):139-42.
- ↑ de Amorim Garcia Filho CA, Gomes AH, de A Garcia Soares AC, de Amorim Garcia CA. Clinical features of 121 patients with diffuse unilateral subacute neuroretinitis. Am. J. Ophthalmol. 2012;153(4):743-9.
- ↑ 14.0 14.1 Gomes AH, Garcia CA, Segundo Pde S, Garcia Filho CA, Garcia AC. Optic coherence tomography in a patient with diffuse unilateral subacute neuroretinitis. Am. J. Ophthalmol. 2012;153(4)743-9.
- ↑ 15.0 15.1 Vezzola D, Kisma N, Robson AG, Holder GE, Pavesio C. Structural and functional retinal changes in eyes with DUSN. Retina. 2014;34(8):1675-82.
- ↑ Audo I, Webster AR, Bird AC, et al. Progressive retinal dysfunction in diffuse unilateral subacute retinitis. Br. J. Ophthalmol. 2006;90:793-4.
- ↑ 17.0 17.1 17.2 Bervel RF, Casella AM, de Souza EC, Farah ME. Evaluation of patients with diffuse unilateral neuroretinitis by spectral domain optical coherence tomography with enhanced depth imaging. Clin. Ophthalmol. 2014;8:1081-7.
- ↑ 18.0 18.1 18.2 Myint K, Sahay R, Mon S, Saravanan VR, Narendran V, Dhillon B. “Worm in the eye”: the rational for treatment of DUSN in south India. Br. J. Ophthalmol. 2006;90(9):1125-7.
- ↑ de Souza EC, Casella AM, Nakashima Y, Moteiro ML. Clinical features and outcomes of patients with diffuse unilateral subacute neuroretinitis treated with oral albendazole. Am. J. Ophthalmol. 2005;140:437-45.
- ↑ 20.0 20.1 Vedantham V, Vats MM, Kakade SJ, et al. Diffuse unilateral subacute neuroretinitis with unusual findings. Am. J. Ophthalmol. 2006;142:880-3.
- ↑ 21.0 21.1 Venkatesan P. Albendazole. J. Antimicrob. Chemother. 1998;41:145-7.
- ↑ Shukla, D, Chakraborty S. Pre-macular nematode in diffuse unilateral subacute neuroretinitis. Eye (Lond). 2008;22(9):1198-200.
- ↑ McDonald HR, Kazacos KR, Schatz H, Johnson RN. Two cases of intraocular infection with Alaria mesocercaria (Trematoda). Am. J. Ophthalmol. 1994;117(4):447-55.
- ↑ Tarantola RM, Elkins KA, Kay CN, Folk JC. Photoreceptor recovery following laser photocoagulation and albendazole in diffuse unilateral subacute neuroretinitis. Arch. Ophthalmol. 2011;129(5):669-71.
- ↑ Natesh S, K H, Nair U, Nair K. Subretinal worm and repeat laser photocoagulation. Middle East Afr. J. Ophthalmol. 2010;17(2):183-5.
- ↑ Gass JDM, Olsen KR. Retina. 3rd edition. St. Louis: Mosby; 2001. 1669-78.
- ↑ Garcia CA, Gomes AH, Vianna RN, Souza Filho JP, Garcia Filho CA, Orefice F. Late-stage diffuse unilateral subacute neuroretinitis: photocoagulation of the worm does not improve the visual acuity of the affected patients. Int. Ophthalmol. 2005;26(1-2):39-42.
- ↑ Garcia CA, Gomes AH, Garcia Filho CA, Vianna RN. Early-stage diffuse unilateral suacute neuroretinitis: improvement of vision after photocoagulation of the worm. Eye (Lond). 2004;18(6)624-7.