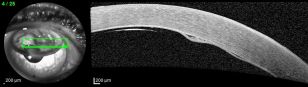
Descemet Membrane Detachment
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Descemet membrane detachment (ICD-9 371.33 – Rupture in Descemet’s membrane)
Disease
Descemet membrane detachment (DMD) is a disease of the posterior cornea that mostly occurs as a complication of intraocular surgery but also due to ocular trauma and even spontaneously. After a DMD, the overlying corneal stroma and epithelium swell, and visual acuity can be permanently affected. Although most DMD resolve spontaneously after surgery, descemetopexy is the gold standard for the management of persistent DMD.
Etiology
DMD normally occurs as aqueous enters the pre-descemetic space along a tear in Descemet’s membrane (DM). The main cause of DMD is iatrogenic, after intraocular surgery, followed by ocular trauma and in rare, predisposed cases, spontaneously.
Cataract surgery is where DMD happens the most, especially after extracapsular cataract extraction and phacoemulsification (with an incidence of 2.5% and 0.5% respectively).[1] DMD has also been reported in other surgeries such as keratoplasty, pars plana vitrectomy, iridectomy, trabeculectomy, laser capsulotomy, laser sclerostomy and viscocanalostomy.[1][2] [3]Chemical injuries (especially alkaline) and blunt trauma (e.g., birth forceps injury) can also cause DMDs. In chemical injuries, the presence of limbal ischemia, iris synechiae and exudations in the anterior chamber seem to play a role in DMD[4]. Spontaneous DMD occurs in acute hydrops associated with ectatic corneal disorders or as a delayed presentation after uncomplicated surgery in predisposed patients.[5][6]
Risk Factors
Various risk factors for DMD can be divided into patient-related, intraoperative, and postoperative factors.
Patient-related risk factors include age over 65 years, preexisting endothelial diseases like Fuchs dystrophy, and intrinsic Descemet stromal interface abnormalities. Previous history of chemical injury, bleeding from corneal neovascularization and dense cataracts. Intraoperative risk factors include use of blunt instruments for incisions, tight or small incisions as compared to the size of the phacoemulsification probe or poorly performed clear corneal incisions (small, oblique, or ragged), inadvertent insertion of surgical instruments between stroma and DM, entry into the anterior chamber in a soft globe, and prolonged surgical time. Postoperative risk factors include genetic causes of weak adhesion between DM and posterior corneal stroma when DMD presents weeks after uncomplicated cataract surgery.[6][3]
Diagnosis[1][6]
The diagnosis of DMD is mostly clinical, based on history and slit lamp examination, especially if the cornea is clear. However, if there is significant corneal edema, imaging tools such as anterior segment optical coherence tomography (AS-OCT) are fundamental for the diagnosis.
History
Most patients will have a history of recent intraocular surgery and since DMDs are not a rare complication, it’s important to discuss predisposing factors with the patient before surgery.
The natural history of DMD is still uncertain and unpredictable. Although most DMDs are small and self-sealing with no corresponding visual complaints, some can present with severe corneal edema and vision loss if the detachment is extensive. In this case, spontaneous resolution is unlikely and if not treated in time, can lead to corneal decompensation and opacification.
Physical Examination
The examination should consist of an ophthalmic work-up including visual acuity, slit-lamp examination, gonioscopy and other diagnostic tests like UBM and AS-OCT.
Signs
DMD is usually seen as a translucent membrane in the anterior chamber, at the site of corneal incision or DM perforation. The hallmark sign of DMD is localized or diffuse corneal edema over the DMD that, with time, can become persistent. Other signs include DM folds and microcystic epithelial edema or bullae. Extensive and central DMDs may create a typical “double anterior chamber” appearance. Any case with severe post operative corneal edema should be investigated to rule out DMD so that timely intervention can be performed.[6]
Symptoms
Patients with DMD present with poor visual improvement after cataract surgery or sudden vision loss in cases with delayed presentation or after keratoplasty and glaucoma surgery. Foreign body sensation can be present due to irregular corneal epithelium.
Clinical diagnosis
The diagnosis of DMD is mostly clinical. If the overlying cornea is clear, a slit-lamp examination can quickly identify DMDs. Gonioscopy may also be used for localized peripheral DMDs.
Diagnostic procedures
In cases where severe corneal edema is present, other diagnostic tests can aid the diagnosis. The most used and accurate exam for the diagnosis of DMDs is the anterior segment optical coherence tomography (AS-OCT). AS-OCT is a quick, non-contact modality that can determine the exact location, configuration, and extent of the DMD, not only confirming the diagnosis but also guiding the treatment method and outcomes.
Other less-used tools are ultrasound biomicroscopy, Scheimpflug imaging and confocal microscopy.
Management
The management of DMD is on a case-by-case basis. Early decision on the therapeutic approach is the most important factor to achieve the best visual outcome. Other important decision-making parameters include the site, extent, and height of the DMD along with the presence of any scrolled edges.[6]
There are many DMD classifications by several authors. In one of them, Kumar et al proposed an AS-OCT-based HELP algorithm (stands for height (measured as distance between detached DM and corneal stroma), extent of DM in the cornea (central, para central and peripheral cornea), length of the detachment and pupil (position of DMD to pupil)) to aid in the management of different types of DMD:[7]
- Conservative treatment:
- All DMDs with a height < 100 µm and length < 1 mm;
- DMDs with a height of 100 – 300 µm and length of 1 – 2 mm not involving the pupil;
- DMDs with a height > 300 µm and length > 2 mm, involving the peripheral cornea (beyond 8 mm of the centre);
- Surgical treatment:
- DMDs with a height of 100 – 300 µm and length of 1 – 2 mm involving the central cornea (5 mm);
- DMDs with a height > 300 µm and length > 2 mm involving the central and paracentral cornea (8 mm)
Medical therapy
There are many reports on the spontaneous resolution of corneal edema, either by reattachment of the DM or migration of nearby endothelial cells.
Conservative treatment includes topical steroids and hyperosmotic agents. Steroids help to control inflammation and reduce the risk of DM fibrosis. Hypertonic agents help to clear corneal edema by dehydrating stroma.
Surgery
Surgical interventions are advocated by many authors in cases of scrolled, nonplanar, extensive and visually impairing DMDs, mostly to hasten visual rehabilitation and prevent fibrosis of DM. Also, all persistent DMD cases with conservative therapy should undergo surgical intervention.
Descemetopexy (or Pneumodescemetopexy):[2][6]
It’s the surgical reattachment of the detached DM with an injection of air and/or isoexpansile gases like perfluoropropane (C3F8, 12 – 14%) and sulfur hexafluoride (SF6, 15 – 20%). Gases reduce the need for reinjection due to their longer reabsorption time (SF6: 2 weeks and C3F8: 6 weeks). It has become the gold standard treatment for DMD management mainly due to its ease of execution and subsequent good outcomes. The technique generally involves filling a syringe with the desired air/gas and injecting it with a 27 – 30G needle. Generally, a complete gas-filled chamber is maintained for 15 – 20 minutes and then release one-third of the bubble to avoid a postoperative pupillary block. It is advised to do an inferior peripheral iridectomy and for the patient to maintain a supine position after the procedure. Other surgeons prefer to use cycloplegic drops and prophylactic peripheral iridotomy to prevent high intraocular pressure.
Sharma et al suggested an AS-OCT-based algorithm for the management of persistent DMD after intraocular surgery. All the planar DMDs involving the superior half of the cornea should undergo intracameral injection of air. The use of gas was recommended for DMD involving the inferior half of the cornea and superior DMD with scrolled edges.[2]
Reattachment outcomes vary from 90% – 100% of cases. Reinjection is necessary for a small percentage of cases (4 -7%) and should be attempted before more complex procedures like endothelial keratoplasty.[8][9][10]
Mechanical tamponade:[1]
Surgical repair with intracameral injection of viscosurgical devices or perfluorocarbons has been reported in isolated case reports with some success but due to intraocular pressure spikes with viscoelastic and possible endothelial toxicity with perfluorocarbons, care should be taken.
Suture fixation:[1]
Transcorneal interrupted suturing using a 10/00 monofilament Nylon suture has been described, frequently associated with intracameral injection of air/gas.
Other techniques:[6]
Manual repositioning of the DM is now considered an obsolete procedure. Various types of descemetotomy have also been reported alone or followed by intracameral injection of air/gas. Keratoplasty is the last treatment option for recalcitrant DMD cases. Descemet Stripping Endothelial Keratoplasty is the preferred technique for the management of these persistent cases.
Complications
The most common complication after Descemetopexy is persistent DMD, followed by raised intraocular pressure and pupillary block glaucoma (up to 13% of cases),[7][11] especially when using C3F8 gas.[12] Other less common complications of Descemetopexy include uveitis[10] and Urrets-Zavalia syndrome due to iris ischemia.[13]
Prognosis
The prognosis of most patients with DMD is good, especially in small, peripheral cases. Prompt treatment should be advised for cases where there is a higher probability of persistent edema like large central DMDs.
See Also
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Chow VWS, Agarwal T, Vajpayee RB, Jhanji V. Update on diagnosis and management of Descemet’s membrane detachment. Curr Opin Ophthalmol. 2013;24(4):356-361. doi:10.1097/ICU.0B013E3283622873
- ↑ Jump up to: 2.0 2.1 2.2 Sharma N, Gupta S, Maharana P, Shanmugam P, Nagpal R, Vajpayee RB. Anterior Segment Optical Coherence Tomography-Guided Management Algorithm for Descemet Membrane Detachment After Intraocular Surgery. Cornea. 2015;34(9):1170-1174. doi:10.1097/ICO.0000000000000514
- ↑ Jump up to: 3.0 3.1 Trindade LC, Attanasio de Rezende R, Bisol T, J Rapuano C. Late Descemet membrane detachment after uneventful cataract surgery. Am J Ophthalmol Case Rep. 2022 Dec 23;29:101783. doi: 10.1016/j.ajoc.2022.101783. PMID: 36632336; PMCID: PMC9827023.
- ↑ Li YT, Wu WY, Li JY, Chan SY, Ang M, Feng Y. Types of Descemet Membrane Detachment After Ocular Surface Burns: The Factor Long Been Ignored. Cornea. 2023 Nov 1;42(11):1426-1431. doi: 10.1097/ICO.0000000000003210. Epub 2022 Dec 12. PMID: 36729715; PMCID: PMC10538613.
- ↑ Basu S, Vaddavalli PK, Vemuganti GK, Hasnat Ali M, Murthy SI. Anterior segment optical coherence tomography features of acute corneal hydrops. Cornea. 2012;31(5):479-485. doi:10.1097/ICO.0B013E318223988E
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Singhal D, Sahay P, Goel S, Asif MI, Maharana PK, Sharma N. Descemet membrane detachment. Surv Ophthalmol. 2020;65(3):279-293. doi:10.1016/j.survophthal.2019.12.006
- ↑ Jump up to: 7.0 7.1 Kumar DA, Agarwal A, Sivanganam S, Chandrasekar R. Height-, extent-, length-, and pupil-based (HELP) algorithm to manage post-phacoemulsification Descemet membrane detachment. J Cataract Refract Surg. 2015;41(9):1945-1953. doi:10.1016/J.JCRS.2015.01.020
- ↑ Chaurasia S, Ramappa M, Garg P. Outcomes of air descemetopexy for Descemet membrane detachment after cataract surgery. J Cataract Refract Surg. 2012;38(7):1134-1139. doi:10.1016/J.JCRS.2012.01.030
- ↑ Fan NW. Outcomes of repeat descemetopexy in post-cataract surgery descemet membrane detachment. Am J Ophthalmol. 2014;157(6):1330-1331. doi:10.1016/J.AJO.2014.02.003
- ↑ Jump up to: 10.0 10.1 Odayappan A, Shivananda N, Ramakrishnan S, Krishnan T, Nachiappan S, Krishnamurthy S. A retrospective study on the incidence of post-cataract surgery Descemet’s membrane detachment and outcome of air descemetopexy. Br J Ophthalmol. 2018;102(2):182-186. doi:10.1136/BJOPHTHALMOL-2016-309766
- ↑ Jain R, Murthy SI, Basu S, Ali MH, Sangwan VS. Anatomic and visual outcomes of descemetopexy in post-cataract surgery descemet’s membrane detachment. Ophthalmology. 2013;120(7):1366-1372. doi:10.1016/J.OPHTHA.2012.12.043
- ↑ Garg J, Mathur U, Acharya MC, Chauhan L. Outcomes of Descemetopexy with Isoexpansile Perfluoropropane after Cataract Surgery. J Ophthalmic Vis Res. 2016;11(2):168-173. doi:10.4103/2008-322X.183932
- ↑ Maurino V, Allan BDS, Stevens JD, Tuft SJ. Fixed dilated pupil (Urrets-Zavalia syndrome) after air/gas injection after deep lamellar keratoplasty for keratoconus. Am J Ophthalmol. 2002;133(2):266-268. doi:10.1016/S0002-9394(01)01308-3