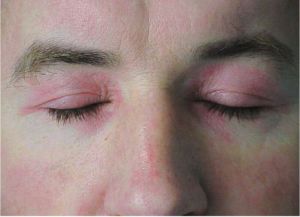
Dermatomyositis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Dermatomyositis is an idiopathic, microangiopathic condition characterized by muscle weakness and skin rash.[1] Dermatomyositis typically presents with symmetric, proximal skeletal muscle weakness. Gottron papules (flat-topped, erythematous to violaceous papules and plaques on bony prominences especially the metacarpophalangeal joints (MCP), the proximal interphalangeal joints (PIP), and the distal interphalangeal joints (DIP) and heliotrope rashes (violaceous periorbital rash) are frequently observed and highly suggestive of dermatomyositis. Ophthalmic manifestations include periorbital heliotrope eyelid eruptions and associated periorbital erythema, edema, ptosis, chemosis, and exophthalmos. Dermatomyositis is thought to be an autoimmune and/or paraneoplastic condition with genetic and environmental factors contributing to the onset of the disease.
1. Disease Entity
Dermatomyositis (DM)
1.1 Disease
DM is an idiopathic, inflammatory autoimmune, paraneoplastic disease of muscle tissue that is characterized by proximal muscle weakness and a characteristic skin rash. DM may have a gradual or acute onset and can be characterized by a waxing and waning progression. Heliotrope eyelid eruptions are considered a hallmark of DM affecting the eye. Other ocular manifestations of DM include conjunctival edema, nystagmus, extraocular muscle weakness, iritis, cotton wool spots, optic atrophy, and conjunctival pseudopolyposis.[2] Retinopathy is also a rare presentation. Eyelid and lens abnormalities are also seen commonly in juvenile DM. Dermatomyositis is highly associated with malignancy in adult patients.[3]
1.2 Etiology
Multiple genetic, immunologic, and environmental factors have been found to be associated with DM. Multiple human leukocyte antigen (HLA) haplotypes are associated with dermatomyositis including HLA-A*68, HLA-DRB1*0301, HLA-DQA1*0104, HLA-DRB1*07, DQA1*05, and DQB1*02. Viruses such as Coxsackie B, enteroviruses, and parvoviruses may be potential triggers for autoimmunity. Antineoplastic medications (e.g., hydroxyurea), antibiotics (e.g., penicillin), nonsteroidal anti-inflammatory drugs (e.g., phenylbutazone and D-penicillamine), statins, and some vaccines have also been investigated as potential triggers of DM. In addition, high intensity ultraviolet (UV) radiation has been found to occur more frequently among women with DM and may be an environmental factor contributing to the etiology.[4] In addition, DM may present secondary to paraneoplastic syndromes in breast cancer and other malignancies.[5]
1.3 Risk Factors
Risk factors, apart from genetic and environmental etiological factors mentioned above, are not well characterized. A case-control study found an association between inhaled pollutants and tobacco smoking during fetal development and juvenile DM.[6] An association with high airborne pollution has been proposed in amyopathic DM.[7] A higher prevalence of DM was noted in Southern Europe compared to Northern Europe and may be due to the increased exposure to UV-B light, other environmental factors, or differences in genetic background.[8] A study in Quebec found a higher prevalence of DM in urban areas.[9]
1.4 General Pathology
DM is believed to be mediated by C3 cleavage to form C3b and C4b, which activates complement, which is deposited in muscle capillaries and the endothelium of arterioles. The activation of complement and deposition on endomysial capillaries causes lysis and muscle ischemia. Subsequently, the membrane attack complex is formed and deposits on vascular walls leading to inflammation and hypoxic injury to the tissue. This results in atrophy of muscle fibers, particularly peripheral fibers that have less collateral vascular supply, which can lead to necrosis and degeneration.[4]
2. Diagnosis
DM is a rare condition that can present with diverse clinical presentations, making it challenging to diagnose. Muscle biopsy on weak muscles, identified by physical exam and/or electromyography, is the most accurate test to confirm the diagnosis of DM for patients who lack skin findings. Skin biopsy should be done for patients who have characteristic skin manifestations but lack muscle weakness.[4] Laboratory studies in DM include creatine kinase, aldolase, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase and erythrocyte sedimentation rate. Myositis-specific antibody (MSA) panels are an emerging assessment tool and include assessment of levels of anti-Jo, anti-Mi2, anti-signal recognition particle (anti-SRP), anti-melanoma differentiation associated gene 5 (anti-MDA5), anti-transcription intermediate factor 1-gamma (anti-TIF-1 gamma), anti-small ubiquitin like modifier-1 activating enzyme (anti-SAE), and anti-nuclear matrix protein 2 (anti-NXP2) antibodies.[10] Electromyography is another diagnostic tool to assess for DM. Findings that are suggestive of DM include increased insertional activity, spontaneous fibrillations, positive sharp waves, complex repetitive discharges, early recruitment, and low-amplitude, short polyphasic motor unit potentials.
If skin manifestations predominate, a skin biopsy can help with diagnosis. Findings are similar to those found in systemic lupus erythematosus (SLE), including vacuolar changes in the basal layer, increased lymphocytic infiltrate, and increased mucin deposition in the dermis.[4]

Ophthalmologic findings, especially heliotrope eyelid eruption, conjunctival edema, nystagmus, extraocular muscle imbalance, and iritis can be used to support the diagnosis.[12] The classic heliotrope rash is named after the heliotrope flower. The rash is characterized by violet or blue rash on the upper eyelids bilaterally and is associated with edema and telengiactesia.[11]
2.1 History
Ernst Wagner was the first person to provide a detailed description of DM in 1863, and Hans Unverricht published a description of DM in 1887.[13] Currently, the diagnosis of DM is made clinically based upon the Gottron papule, heliotrope rash and other characteristic findings.
2.2 Physical examination
Comprehensive history and physical exam can be performed to identify the type of muscular and cutaneous symptoms, to assess for other organ system involvement (especially respiratory, cardiac and esophageal) and to assess for malignancies, which are commonly associated with DM. Ocular physical exam findings include dilated vessels of the bulbar conjunctiva, iritis, episcleritis, uveitis with glaucoma, cotton wool spots in the retina, and in rare cases, Elschnig spots.[2] Later in disease progression, diffuse pallor of the optic disc is occasionally observed as a result of retinal neuronal atrophy.[2] Cotton wool spots are a nonspecific exam finding indicative of arteriolar obstruction or capillary damage as a result of nerve fiber layer infarction producing axonal swelling and rupture.[2] Pachymetry measurements and corneal volumes in DM have been noted to be significantly lower than control patients and there is a higher prevalence of dry eye.[14] Bilateral periorbital masses of extensive mucin deposition have been described in DM.[12] DM can also cause central retinal artery occlusion.[15] Ophthalmoplegia, diplopia and strabismus may also occur in DM.[2]
Proximal muscle weakness, especially in the hip flexors, deltoids, and neck flexors is a common physical exam finding. Distal muscle involvement, including ocular muscle involvement, is an atypical presentation. Muscle tenderness, depressed deep tendon reflexes, and muscle atrophy can be seen at later stages of dermatomyositis.
Skin rashes may begin before or concurrently with muscular symptoms. Pathognomonic findings include Gottron papules and heliotrope rashes. Other signs that can help distinguish dermatomyositis from other diseases with skin symptoms include Gottron sign, facial erythema, shawl sign, V-sign, poikiloderma of Civatte, Holster sign, periungual involvement, mechanic’s hand, scalp involvement, and calcinosis cutis. In addition, patients may present with nail changes, alopecia, changes in pigmentation, pruritis, photosensitivity, and a wide range of rashes. Skin changes may occur prior to muscular symptom onset, or concurrently.[4]
Physical exam may also identify non-erosive polyarthritis or arthralgia of the small joints of the hands, joint pain, or swelling. Respiratory findings may include exertional dyspnea, exercise intolerance, non-productive cough, dry crackles, and reduced movement of the thorax upon respiration. Esophageal findings may include weakness of the oropharynx and upper esophagus and GERD. Other physical exam findings include Raynaud’s phenomenon, ulcers, cardiac symptoms, fever, malaise, and weight loss.[4]
2.3 Signs
Signs of DM include symmetric proximal skeletal muscle weakness, Gottron papules and heliotrope rash.[4] Heliotrope eyelid eruptions, conjunctival edema, nystagmus, extraocular muscle imbalance, iritis, cotton wool spots, optic atrophy, and conjunctival pseudopolyposis are ocular signs of DM.
2.4 Symptoms
Symptoms of DM include difficulty climbing stairs, rising from a seated position, lifting objects, combing hair, raising head from pillow, and in severe cases dysphagia or dysphonia. Cutaneous symptoms include several types of skin rashes, photosensitivity, changes in pigmentation, and pruritus.[4] Ophthalmic symptoms includes heliotrope eyelid eruptions, periorbital redness, periorbital edema, ptosis, chemosis, exophthalmos, ophthalmoplegia, diplopia and strabismus.
2.5 Clinical diagnosis
Due to the idiopathic etiology, clinical diagnosis is difficult and requires extensive testing to rule out other potential causes of symptoms. Assessment of laboratory, skin manifestations, muscle symptoms, history of symptoms, biopsies, electromyograms (EMG), and rheumatologic MSA panels can be used to support a clinical diagnosis of dermatomyositis.[4] A serum antinuclear antibody (ANA) level may be checked as ANA negativity has been associated with increased likelihood of concomitant malignancy.[16]
Management
3.1 General Treatment
Current treatment is a combination of pharmacologic treatments and patient education, including systemic glucocorticoids, immunosuppressive therapy, intravenous immunoglobulin therapy (IVIG), physical therapy, symptomatic treatment of cutaneous symptoms, and patient education.[4]
First-line treatment of dermatomyositis with muscular symptoms is glucocorticoids until muscle strength improves and muscle enzyme levels decline. Immunosuppressant drug therapy, e.g. azathioprine or methotrexate, can be co-administered with steroid therapy in order to reduce and alleviate steroid-related adverse effects, such as cushingoid effects and secondary diabetes.[4] Anti-resorptive therapy (e.g., bisphosphonates) may be helpful for patients who are at high risk for osteoporosis. Antibiotics, such as trimethoprim sulfamethoxazole, may be considered prophylactically for Pneumocystis jirovecii.[17] Currently, intravenous immunoglobulin therapy is a potential future direction of therapy.[15] Intravenous immunoglobulin therapy has been used in refractory DM with some notable successful response.[17]
With regards to ophthalmologic preventative care, alterations of the local environment, patient education, dietary modification including fatty acid supplementation, addition of ocular lubricants of various types, and lid hygiene can be considered if dry eye disease symptoms are present.[18] In addition, prompt ophthalmology referral for management of disease course can be considered to limit progression of ophthalmic manifestations.[19]
Patients diagnosed with dermatomyositis should undergo age-specific malignancy workup, which may include colonoscopy (or fecal occult blood test), urine analysis, mammography, and pap smear.[3]
3.2 Surgery
Surgery is not typical for DM however gastrotomy may be helpful for patients whose esophageal dysfunction has become severe, and surgical removal of calcinotic nodules if present may be done.[4]
3.3 Prognosis
Upon the initiation of treatment, visual recovery is usually complete, and typically hemorrhages and cotton wool spots resolve.[2] Mortality rate of DM is 10%, and is commonly caused by malignancy, pulmonary complications, and ischemic cardiomyopathy.[4] Advanced age, initiation of treatment more than six months after onset of symptoms, severe muscle weakness, dysphagia, pulmonary dysfunction, cardiovascular dysfunction, and underlying malignancy indicate higher mortality and poorer outcomes. 65% of patients who survive have normal strength, 34% have mild disability, and 16% have no disability. 20% of patients achieve remission, 80% have a chronic course.[4]
3.4 Summary
DM is an idiopathic, microangiopathic myopathy characterized by muscle weakness, Gottron papules and heliotrope rashes, and has a multi-factorial pathogenesis with multiple genetic, immunologic, and environmental factors that contribute.[1] Diagnosis can be supported by ophthalmologic examination of heliotrope eyelid eruptions and associated periorbital erythema, edema, ptosis, chemosis, and exophthalmos. Ophthalmologic management of dry eye, lid hygiene, and preventative management to slow progression is current treatment practice. Innovative treatments for dermatomyositis’ ophthalmic manifestations is a future direction worth interrogating.
4. References
- ↑ Jump up to: 1.0 1.1 Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003 Sep 20;362(9388):971-82. doi: 10.1016/S0140-6736(03)14368-1. PMID: 14511932.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 Dourmishev, L. A., & Durmishev, A. (2009). Ophthalmic Complications of Dermatomyositis. In Dermatomyositis: Advances in recognition, understanding, and management (pp. 93-95). Berlin: Springer. doi:10.1007/978-3-540-79313-7
- ↑ Jump up to: 3.0 3.1 Qudsiya Z, Waseem M. Dermatomyositis. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558917
- ↑ Jump up to: 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 Qudsiya Z, Waseem M. Dermatomyositis. 2020 Aug 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID: 32644343.
- ↑ Sandhu, N. P., Zakaria, S., Degnim, A. C., & Boughey, J. C. (2011). Dermatomyositis presenting as a paraneoplastic syndrome due to UNDERLYING breast cancer. Case Reports, 2011(Jan29 1). doi:10.1136/bcr.10.2010.3416
- ↑ Orione, M.A.M., Silva, C.A., Sallum, A.M.E., Campos, L.M.A., Omori, C.H., Braga, A.L.F. and Farhat, S.C.L. (2014), Risk Factors for Juvenile Dermatomyositis: Exposure to Tobacco and Air Pollutants During Pregnancy. Arthritis Care & Research, 66: 1571-1575. https://doi.org/10.1002/acr.22358
- ↑ Pearson, D. R., & Werth, V. P. (2019). Geospatial correlation of amyopathic dermatomyositis with fixed sources of airborne pollution: A retrospective cohort study. Frontiers in Medicine, 6. doi:10.3389/fmed.2019.00085
- ↑ Hengstman GJ, van Venrooij WJ, Vencovsky J, Moutsopoulos HM, van Engelen BG. The relative prevalence of dermatomyositis and polymyositis in Europe exhibits a latitudinal gradient. Ann Rheum Dis. 2000 Feb;59(2):141-2.
- ↑ Bernatsky S, Joseph L, Pineau CA, Bélisle P, Boivin JF, Banerjee D, Clarke AE. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: age, sex and regional differences. Ann Rheum Dis. 2009 Jul;68(7):1192-6.
- ↑ Waldman, R., DeWane, M. E., & Lu, J. (2020). Dermatomyositis: Diagnosis and treatment. Journal of the American Academy of Dermatology, 82(2), 283-296. doi:10.1016/j.jaad.2019.05.105
- ↑ Jump up to: 11.0 11.1 Fiorentino, D., Marvi, U., & Chung, L. (2012). Clinical presentation and evaluation of dermatomyositis. Indian Journal of Dermatology, 57(5), 375. doi:10.4103/0019-5154.100486
- ↑ Jump up to: 12.0 12.1 Mina Z Al-Awqati, Olayemi Sokumbi, Katherine G Gold, Florentina Berianu, Periorbital masses in dermatomyositis, Rheumatology, Volume 59, Issue 12, December 2020, Pages e136–e137, https://doi-org.srv-proxy1.library.tamu.edu/10.1093/rheumatology/keaa421
- ↑ Levine, T. D. (2003). History of dermatomyositis. Archives of Neurology, 60(5), 780. doi:10.1001/archneur.60.5.780
- ↑ Griger Z, Danko K, Bodoki L, Aszalos Z, Nemeth G, Ziad H, Gesztelyi R, Zsuga J, Szodoray P, Kemeny-Beke A. Corneal Involvement of Patients with Polymyositis and Dermatomyositis. Ocul Immunol Inflamm. 2020;28(1):58-66. doi: 10.1080/09273948.2018.1547407. Epub 2018 Nov 16. PMID: 30444429
- ↑ Jump up to: 15.0 15.1 Şimşek C, Doğru M, Kojima T, Tsubota K. Current Management and Treatment of Dry Eye Disease. Turk J Ophthalmol. 2018 Dec 27;48(6):309-313. doi: 10.4274/tjo.69320. PMID: 30605938; PMCID: PMC6330664.
- ↑ Hoesly PM, Sluzevich JC, Jambusaria-Pahlajani A, Lesser ER, Heckman MG, Abril A. Association of antinuclear antibody status with clinical features and malignancy risk in adult-onset dermatomyositis. J Am Acad Dermatol. 2019 May;80(5):1364-1370. doi: 10.1016/j.jaad.2018.11.023. Epub 2018 Nov 17. PMID: 30458207.
- ↑ Jump up to: 17.0 17.1 Pongratz D. Therapeutic options in autoimmune inflammatory myopathies (dermatomyositis, polymyositis, inclusion body myositis). J Neurol. 2006 Sep;253 Suppl 5:V64-5. doi: 10.1007/s00415-006-5010-2. Erratum in: J Neurol. 2008 Feb;255(2):308. PMID: 16998756 14.
- ↑ Cafardi, J. M., & Sami, N. (2015). Intravenous immune Globulin in AMYOPATHIC Dermatomyositis - report of two cases and review of the literature. The Open Rheumatology Journal, 9(1), 77-81. doi:10.2174/1874312901409010077
- ↑ Sharma M, Prashar A, Tuli R, Sharma RK, Mahajan VK. Atypical central retinal artery occlusion: an uncommon cause of retinopathy and visual loss in dermatomyositis. Int J Rheum Dis. 2019 Feb;22(2):325-330. doi: 10.1111/1756-185X.12750. Epub 2015 Sep 25. PMID: 26403216.