Cyclophotocoagulation in Pediatric Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Management of pediatric glaucoma remains particularly challenging for a variety of reasons, including differences in tissue behavior from adults such as highly active healing and scarring, the potential for amblyopia and long-term implications, and difficulties in post-operative course. [1][2][3] Pediatric glaucoma can be due to a primary cause like primary congenital glaucoma or juvenile open angle glaucoma (JOAG), or can develop secondary to surgery, trauma or systemic disease such as Sturge-Weber Syndrome.[4] As opposed to adult glaucoma, primary congenital glaucoma is largely a surgical disease. Preferred initial management is angle surgery such as goniotomy[5] or trabeculotomy ab externo.[6] Filtering surgery via trabeculectomy[7] or placement of glaucoma drainage devices[8][9][10] are typically used as secondary options after failure of angle surgery.
Cyclodestructive procedures, such as cyclocryotherapy[11] and cyclophotocoagulation (CPC)[12][13], have traditionally been utilized in pediatric glaucoma refractory to all other medical and surgical treatments, eyes with limited visual potential, eyes with silicone oil tamponade, and eyes with uncontrolled intraocular pressure (IOP).[14] However, the development of micropulse transscleral CPC (MP-TSCPC) has led to the possibility of using cyclodestructive procedures earlier in the disease course due to its lower side-effect profile compared to traditional continuous wave transscleral CPC (CW-TSCPC).[15] Traditionally, CW-TSCPC has been the mainstay of cyclodestruction in pediatric glaucoma.[16][17] Endoscopic cyclophotocoagulation can also be used for a more targeted approach in pseudophakic and aphakic patients as an alternative to transscleral therapy.[18][19]
Transscleral Cyclophotocoagulation
Surgical Technique
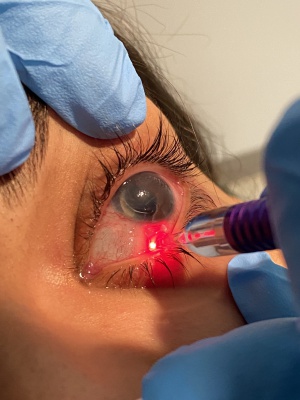
Transscleral cyclophotocoagulation (TSCPC) is a non-invasive cyclodestructive procedure using lasers that emit in the near infrared spectrum delivered by a probe placed externally on the sclera. Laser options include the Neodymium:yytrium-aluminum-garnet (Nd:YAG) laser in contact fashion at 1064 nm wavelength or the semiconductor diode laser at 810 nm wavelength. Due to its greater absorption by melanin in the ciliary epithelium, the diode laser system is currently the standard modality for refractory glaucoma in children.[14][20][21] Depending on the child’s age, general anesthesia along with retrobulbar or peribulbar anesthesia is advised. A lid speculum is placed for optimal viewing of the peri-limbal area. Transillumination may be used to locate the ciliary body in eyes with abnormal anatomy particularly if buphthalmic. A specialized handpiece delivers the laser energy posterior to the limbus in either a continuous (G-probe, Iridex, Mountain View, CA) or micropulse (MP3, Iridex, Mountain View, CA, or SubCyclo Vitra 810, Quantel Medical Instruments, Cournon d'Auvergne Cedex, France) fashion. The duration and power of laser energy depends on the method used; common methods are outlined below. After the procedure, topical anti-inflammatory medications such as steroid drops and cycloplegics are given. Alternatively, a steroid subconjunctival injection may be used.
Continuous Wave Transscleral Cyclophotocoagulation
CW-TSCPC is the traditional and most researched method of cyclophotocoagulation. In the standard treatment protocol, initial power is set to 1000-2000 mW for a duration of 2 seconds. Power is increased in 100-250 mW increments until an audible popping sound is heard, caused by a uveal micro-explosion. A lower power is usually required in the pediatric population compared to adults[14]. The power is then titrated down to a level just below which an audible pop could be heard for the remainder of the procedure. The number of application spots is generally 4-8 spots per quadrant, with application to 2-4 quadrants and sparing of the 3:00 and 9:00 positions.
Studies on outcomes for CW-TSCPC in the pediatric population have varied results. 6 papers dating back to 1997 report diode laser CW-TSCPC success rates between 18% and 51%. The average follow-up time in these studies ranged between 10.3 and 60 months.[13][16][17][22][23][24] CW-TSCPC has not been shown to decrease medication burden in the pediatric population.[14] Interestingly, a short-term spike in IOP within 3 months of the procedure has been described to occur more commonly in phakic and younger patients.[16] One study found over 21% of CW-TSCPC cases demonstrated this spike.[22] The need for retreatment after initial CW-TSCPC is high, ranging from 33% to 70%.[13][16][17][22][23][24] CW-TSCPC remains an ancillary treatment for pediatric glaucoma due to the risk of severe complications, including suprachoroidal hemorrhage, retinal detachment, choroidal detachment, hypotony and phthisis. The rate of serious complications in the pediatric population ranges from 2%-39% in the literature.[13][16][17][22][23][24] Complication rates are increased after multiple surgeries and in younger patients.[14] Continuous wave CPC was also shown to have similar efficacy and complication rates to sequential tube shunt surgery following primary tube shunt failure in children.[25]
Intra-operative Transillumination
One of the challenges with TSCPC in pediatric glaucoma is the often distorted limbal anatomy in these patients. One way to better visualize ciliary body location is through some form of transillumination. This can be achieved using the G-probe Illuminate device which incorporates a light into the laser delivery probe. Recently, a low-cost torchlight method of transillumination was described for pediatric cyclophotocoagulation. This method utilizes a simple handheld torchlight directed towards the cornea, creating transillumination of the eye. This method allows for the visualization of the ciliary body, aiding in the precise targeting of the laser treatment during cyclophotocoagulation procedures.
In a recent prospective interventional study conducted in 2023 evaluating diode laser transscleral cyclophotocoagulation (TSCPC) with trans-corneal transillumination using a low-cost torchlight method in refractory pediatric glaucoma, the one-year outcomes were assessed. The study aimed to compare outcomes of the TSCPC procedure with transillumination (TSCPC-TI) against a historical control group that underwent TSCPC without transillumination (TSCPC-No TI) at the one-year follow-up. Results were derived from 42 eyes of 35 patients in the TSCPC-TI group and 31 eyes of 21 patients in the TSCPC-No TI group. The TSCPC-TI group required significantly lower laser energy (24.7±7.8 J) compared to the TSCPC-No TI group (47.2±10.9 J, P<0.0001). Both groups showed a notable reduction in mean topical antiglaucoma medication requirement after treatment, with no statistically significant difference between the groups (P=0.15). However, the TSCPC-TI group exhibited a significantly reduced dependence on oral acetazolamide at the one-year follow-up compared to the TSCPC-No TI group (5.7% vs. 61.9%, P<0.001%). Importantly, no adverse events such as hypotony or choroidal detachment were reported in either group. The study concluded that TSCPC aided by transillumination using a low-cost torchlight method demonstrated effectiveness at one year by reducing intraocular pressure and the need for medication in pediatric refractory glaucoma cases, without observing significant adverse events.[26]
Micropulse Transscleral Cyclophotocoagulation
For MP-TSCPC, the most common setting is to start with a power of 2000 mW applied for 90-180 seconds of treatment time. The laser is applied in a typical “duty cycle” of 31.3%, which delivers the laser in repetitive pulses in a cycle of .5 ms ‘on time’ and 1.1 ms ‘off time’[27][28][29] The MP3 handpiece is held with steady pressure and is swept along the limbus in a painting fashion for the duration of the treatment in the desirable number of quadrants. The power, time, sweeping speed, and treatment of quadrants or hemispheres are all variables that can be modified. The cycling of laser energy in micropulse limits the temperature increase in the tissue, which prevents coagulation necrosis and limits damage to adjacent tissues. Since this procedure is typically not cyclodestructive, a name change to Micropulse Transscleral Laser Treatment (MP-TLT) has been suggested.
Abdelraman et al. compared outcomes of MP-TSCPC to CW-TSCPC in a cohort of 45 eyes of 36 children over the course of 10 months and showed a MP-TSCPC success rate of 71% at 6 months follow-up with a 0% retreatment rate. Compared to CW-TSCPC, the MP-TSCPC group had a higher success rate (71% vs 41%) and lower rate of complications, although neither difference was statistically significant.[20] Elheifney et al showed a 61% success rate using MP-TSCPC with an average follow-up of 15.1 months, and a retreatment rate of 67% during the study and no complications.[30] Lee et al showed a lower success rate of 22% in the pediatric population, potentially due to a different patient population (44% Sturge Weber Syndrome patients) and more stringent definition of success. There were no serious complications during the study.[31] Fam et al also compared MP-TSCPC to CW-TSCPC in children.[32] There was a larger decrease in IOP in the CW-CPC group, although this group had a higher mean baseline IOP. Retreatment rates for MP-CPC was 46% and was 28% for CW-CPC, and there were no reported complications. While some studies suggest a similar efficacy between MP-TSCPC and CW-TSCPC, there is not a consensus in the literature that this is the case. MP-TSCPC does appear to consistently decrease the risk of complications in the pediatric population.
In a recent retrospective cohort study conducted in 2023 comparing MP-CPC and CW-CPC in pediatric glaucoma patients, 28 patients (81 eyes) were analyzed. Both treatment groups were well-matched in age (Mean±SD; 1.76±1.69 vs. 1.56±2.49 years). The study found that patients undergoing MP-CPC experienced significant reductions in IOP at 1, 3, and 6-month intervals, achieving a 22% success rate (SR) at 12 months. Similarly, CW-CPC demonstrated a significant IOP decrease at all follow-up points, with a 27% SR at 12 months. Notably, while the CW-CPC group exhibited a significantly larger overall IOP reduction compared to MP-CPC at the 12-month follow-up (7.99±7.95 vs. 1.78±6.89, P<0.05), the difference in SR between the treatment groups at 12 months was not statistically significant (P>0.05). Importantly, both MP-CPC and CW-CPC showed minimal complications, suggesting comparable safety profiles. The study concluded that both treatments displayed short-term efficacy in reducing IOP, but the effects reverted to near baseline levels after 12 months.[33]
Endoscopic Cyclophotocoagulation
Surgical Technique
Endoscopic CPC combines a laser system, video camera, and illumination probe to allow for direct visualization and targeted treatment of the ciliary body processes while potentially sparing surrounding tissues.[34] A triple function G-probe handpiece (Endo Optiks Inc, Little Silver, NJ) combines a semiconductor diode laser system at 810 nm wavelength, fiberoptic microendoscope, and a Xenon-arc light source. The endoscope has a 110-degree field of view and a depth of focus of 0-20mm. The diode laser has an adjustable power output up to 1.2 watts and is focused using a helium-neon aiming beam at 670 nm wavelength. After the child receives general anesthesia, the surgeon accesses the ciliary body through either a limbal or pars plana incision. In pseudophakic, and aphakic eyes, the ciliary body is typically accessed anteriorly through a corneal incision and with viscoelastic used to inflate the ciliary sulcus.[34] In phakic eyes, endoscopic access is not possible in most cases anteriorly and is high risk for lens damage and cataract formation. As such, phakic eyes are not considered for this approach. Laser energy is delivered continuously in timed pulses of .5-2 seconds ranging from 200-300 mW in power. The treatment endpoint is a whitening and shrinkage of the ciliary processes. Power is titrated down or duration of treatment is shortened if tissue disruption or audible “pops” are visualized. Treatments are applied for 90-360 degrees of the ciliary body or to the extent that is visible through the endoscope being introduced through one or more corneal incisions for access.[19] Post-operatively, antibiotic and anti-inflammatory drops are given and patients are followed-up the next day.
Outcomes
Endoscopic CPC is a preferred surgical method for pseudophakic and aphakic patients and in patients with distorted anatomical landmarks.[18][19][23][35][36] A retrospective review by Neely et al of 51 procedures in 29 children with mean follow-up time of 20 months demonstrated successful IOP reduction <21 mmHg in 43% of patients.[18] Another study by Carter et al examining outcomes in pseudophakic and aphakic patients demonstrated an overall success rate of 53%.[19] When utilized as a primary surgical treatment for glaucoma by Kraus et al, however, endoscopic CPC demonstrated a lower rate of IOP reduction <21 mmHg (46% vs. 55%) and higher treatment failure rate (54% vs. 45%) compared to transscleral CPC as primary treatment.[23] In the most recent studies, Cantor et al showed a success rate of 54%, with 62% of eyes needing only one treatment,[35] and Glaser et al showed a success rate of 64%.[36]
A recent literature review assessed transscleral CW-CPC (CW-TSCPC), endoscopic CPC (ECP), and MP-TLT including both adult and pediatric patients to comprehensively evaluate these procedures. The literature reports highly variable success rates in CW-TCPC, influenced by factors such as the definition of success, underlying glaucoma type, energy settings, follow-up duration, and retreatment rates.[37] Notably, CW-CPC often necessitates repeated sessions, particularly in pediatric patients, and carries risks of inflammation and severe complications such as chronic ocular hypotony or phthisis leading to irreversible visual impairment. CW-TSCPC has primarily found utility in severe glaucoma cases, especially in eyes with limited visual potential, severe pain, or following failed filtering surgeries. Published data regarding ECP are limited but indicate overall favorable success rates and demonstrates relatively low complication rates. However, larger studies with extended follow-up durations are warranted. Despite limited data on MP-TLT's mechanism of action and laser setting standardization, initial findings from diverse case series suggest comparable efficacy and a superior safety profile in the medium term compared to CW-TSCPC.
Complications/Adverse Events
Complications of endoscopic CPC are similar to other forms of CPC and include hypotony, phtisis, retinal detachment, intraocular inflammation, and decrease in vision. Reported complication rates in the literature range from 6% - 11%.[18][19][23][35][36] In addition, infection is a risk due to its invasive nature and the need for an incision. In phakic patients there is also risk of damaging the crystalline lens during the surgical procedure which will lead to cataract formation and the need for additional surgery.[34] A case-study illustrates a rare instance of proliferative vitreoretinopathy (PVR) occurring in a young patient with aphakic glaucoma following transscleral diode laser cyclophotocoagulation, suggesting a potential link between the destruction of the ciliary body and the inflammatory response triggering PVR, emphasizing the importance of postoperative monitoring and prompting further research into this uncommon complication.[38]
A retrospective cohort study conducted in 2022 aimed to examine the occurrence and outcomes of microbial keratitis (MK) following cyclophotocoagulation (CPC) used for treating refractory childhood glaucoma (CG) over six years at a single center. Among 312 children who underwent CPC during the study period, 37 eyes of 33 children developed MK, indicating an incidence of 1.8%. The median interval between CPC and MK occurrence was 4 years. Notably, 20 eyes showed no pain at the onset of MK. The most common microbial isolates were Streptococcus pneumoniae and Staphylococcus epidermis. MK resolved in 46% of the affected eyes after treatment; however, 22% required evisceration or developed phthisis, and keratoplasty failed in 16% of cases. The study highlighted a negative association between the absence of pain at MK onset and the likelihood of resolution after treatment, suggesting a potential link to neurotrophic keratitis following CPC and indicating a poorer response to management strategies.[39]
Conclusion
Cyclophotocoagulation has become a common surgical technique to treat pediatric glaucoma refractory to medications and other surgeries. Transscleral CPC is a noninvasive procedure that can be performed in a continuous wave or micropulse fashion. Comparatively, CW-TSCPC may be slightly more efficacious with a lower retreatment rate, however MP-TSCPC has demonstrated a lower rate of serious complications in pediatric patients likely due to the lower overall energy delivered with decreased coagulation necrosis. Due to this improved safety profile, it is thought MP-TSCPC could be used as a primary surgical glaucoma treatment earlier in the disease course however clinical data are necessary to validate this approach. Endoscopic cyclophotocoagulation is an invasive procedure that allows very targeted treatment and should be considered for pseudophakic and aphakic pediatric patients. To date, no randomized, controlled clinical trials have been conducted on either micropulse or endoscopic CPC and current hypotheses on increased efficacy of these procedures versus standard continuous wave CPC are not based on rigorous experimental or clinical data. Additional comparative protocol studies are needed to determine optimal and safe laser parameters for children.
References
- ↑ Chang I, Caprioli J, Ou Y. Surgical Management of Pediatric Glaucoma. Dev Ophthalmol. 2017;59:165-178.
- ↑ Yu Chan JY, Choy BN, Ng AL, Shum JW. Review on the Management of Primary Congenital Glaucoma. J Curr Glaucoma Pract. 2015;9(3):92-99.
- ↑ Malik R, AlDarrab A, Edward DP. Contemporary management of refractory pediatric glaucoma. Curr Opin Ophthalmol. 2020;31(2):123-131.
- ↑ Thau, A. , Lloyd, M. , Freedman, S. , Beck, A. , Grajewski, A. & Levin, A. V. (2018). New classification system for pediatric glaucoma. Current Opinion in Ophthalmology, 29 (5), 385-394. doi: 10.1097/ICU.0000000000000516.
- ↑ Hassanein DH, Awadein A, Elhilali H. Factors associated with early and late failure after goniotomy for primary pediatric glaucoma. Eur J Ophthalmol. 2020;30(1):162-167.
- ↑ McPherson SD Jr, Berry DP. Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol. 1983;95(4):427-431.
- ↑ Jayaram H, Scawn R, Pooley F, et al. Long-Term Outcomes of Trabeculectomy Augmented with Mitomycin C Undertaken within the First 2 Years of Life. Ophthalmology. 2015;122(11):2216-2222.
- ↑ Chen A, Yu F, Law SK, Giaconi JA, Coleman AL, Caprioli J. Valved Glaucoma Drainage Devices in Pediatric Glaucoma: Retrospective Long-term Outcomes. JAMA Ophthalmol. 2015;133(9):1030-1035.
- ↑ Tai AX, Song JC. Surgical outcomes of Baerveldt implants in pediatric glaucoma patients. J AAPOS. 2014;18(6):550-553.
- ↑ Cunliffe IA, Molteno AC. Long-term follow-up of Molteno drains used in the treatment of glaucoma presenting in childhood. Eye (Lond). 1998;12(Pt 3a):379-385.
- ↑ Wagle NS, Freedman SF, Buckley EG, Davis JS, Biglan AW. Long-term outcome of cyclocryotherapy for refractory pediatric glaucoma. Ophthalmology. 1998;105(10):1921-1927.
- ↑ Phelan MJ, Higginbotham EJ. Contact transscleral Nd:YAG laser cyclophotocoagulation for the treatment of refractory pediatric glaucoma. Ophthalmic Surg Lasers. 1995;26(5):401-403.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 Autrata R, Rehurek J. Long-term results of transscleral cyclophotocoagulation in refractory pediatric glaucoma patients. Ophthalmologica. 2003;217(6):393-400.
- ↑ Jump up to: 14.0 14.1 14.2 14.3 14.4 Souissi, S., Le Mer, Y., Metge, F., Portmann, A., Baudouin, C., Labbé, A. and Hamard, P. (2021), An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma. Acta Ophthalmol, 99: e621-e653. https://doi.org/10.1111/aos.14661
- ↑ Elhefney EM, Mokbel TH, Hagras SM, et al. Micropulsed diode laser cyclophotocoagulation in recurrent pediatric glaucoma. Eur J Ophthalmol. 2020;30(5):1149–1155. doi:10.1177/1120672119858226.
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. Ophthalmology. 2002;109(2):316-323.
- ↑ Jump up to: 17.0 17.1 17.2 17.3 Bock CJ, Freedman SF, Buckley EG, Shields MB. Transscleral diode laser cyclophotocoagulation for refractory pediatric glaucomas. J Pediatr Ophthalmol Strabismus. 1997;34(4):235-239.
- ↑ Jump up to: 18.0 18.1 18.2 18.3 Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS. 2001;5(4):221-229.
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 Carter BC, Plager DA, Neely DE, Sprunger DT, Sondhi N, Roberts GJ. Endoscopic diode laser cyclophotocoagulation in the management of aphakic and pseudophakic glaucoma in children. J AAPOS. 2007;11(1):34-40.
- ↑ Jump up to: 20.0 20.1 Abdelrahman AM, El Sayed YM. Micropulse Versus Continuous Wave Transscleral Cyclophotocoagulation in Refractory Pediatric Glaucoma. J Glaucoma. 2018;27(10):900-905.
- ↑ Quigley HA. Improved Outcomes for Transscleral Cyclophotocoagulation Through Optimized Treatment Parameters. J Glaucoma. 2018;27(8):674-681.
- ↑ Jump up to: 22.0 22.1 22.2 22.3 Hamard P, May F, Quesnot S & Hamard H (2000): La cyclophotocoagulation transsclérale au laser diode dans le traitement des glaucomes réfractaires du sujet jeune. J Fr Ophtalmol 23: 773–780.
- ↑ Jump up to: 23.0 23.1 23.2 23.3 23.4 23.5 Kraus CL, Tychsen L, Lueder GT & Culican SM (2014): Comparison of the effectiveness and safety of transscleral cyclophotocoagulation and endoscopic cyclophotocoagulation in pediatric glaucoma. J Pediatr Ophthalmol Strabismus 51: 120–127.
- ↑ Jump up to: 24.0 24.1 24.2 Fieß A, Shah P, Sii F et al. (2017): Trabeculectomy or transscleral cyclophotocoagulation as initial treatment of secondary childhood glaucoma in Northern Tanzania. J Glaucoma 26: 657–660.
- ↑ Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma. J AAPOS. 2009;13(4):379-383.
- ↑ 1. Choudhary S, Snehi S, Singh A, Thattaruthody F, Pandav SS, Kaushik S. Diode Laser Transscleral Cyclophotocoagulation With A Novel Low-Cost Torchlight Method Of Trans-Corneal Transillumination In Refractory Paediatric Glaucoma. J Glaucoma. 2023 Aug 25. doi: 10.1097/IJG.0000000000002299. Epub ahead of print.
- ↑ Garcia GA, Nguyen CV, Yelenskiy A, et al. Micropulse transscleral diode laser cyclophotocoagulation in refractory glaucoma: short-term efficacy, safety, and impact of surgical history on outcomes. Ophthalmol Glaucoma 2019; 2:402–412.
- ↑ Yelenskiy A, Gillette TB, Arosemena A, et al. Patient outcomes following micropulse transscleral cyclophotocoagulation: intermediate-term results. J Glaucoma 2018; 27:920–925.
- ↑ Zaarour K, Abdelmassih Y, Arej N, et al. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma 2019; 28:270–275
- ↑ Elhefney EM, Mokbel TH, Hagras SM, Al Nagdy AA, Ellayeh AA, Mohsen TA & Gaafar WM (2019): Micropulsed diode laser cyclophotocoagulation in recurrent pediatric glaucoma. Eur J Ophthalmol. 104: 1011–1016.
- ↑ Lee JH, Shi Y, Amoozgar B, Aderman C, De Alba Campomanes A, Lin S & Han Y (2017): Outcome of micropulse laser transscleral cyclophotocoagulation on pediatric versus adult glaucoma patients. J Glaucoma 26: 936–939
- ↑ Anthony Fam, Sarangdev Vaidya, Albert S Khouri; A Comparison of Micropulse and Continuous Wave Cyclophotocoagulation in the Treatment of Refractory Pediatric Glaucoma. Invest. Ophthalmol. Vis. Sci. 2020;61(7):5230.
- ↑ 1. Vega-Garces M, Uppuluri S, Oydanich M, Khouri AS. Comparison of Efficacy of Micropulse and Continuous Wave Cyclophotocoagulation in Pediatric Glaucoma Patients. J Glaucoma. 2023 Nov 3. doi: 10.1097/IJG.0000000000002335. Epub ahead of print.
- ↑ Jump up to: 34.0 34.1 34.2 Pastor SA, Singh K, Lee DA, et al. Cyclophotocoagulation: a report by the American Academy of Ophthalmology. Ophthalmology. 2001;108(11):2130-2138.
- ↑ Jump up to: 35.0 35.1 35.2 Cantor AJ, Wang J, Li S, Neely DE & Plager DA (2018): Long-term efficacy of endoscopic cyclophotocoagulation in the management of glaucoma following cataract surgery in children. J AAPOS 22: 188–191.
- ↑ Jump up to: 36.0 36.1 36.2 Glaser TS, Mulvihill MS & Freedman SF (2019): Endoscopic cyclophotocoagulation (ECP) for childhood glaucoma: a large single-center cohort experience. J AAPOS 23: 84.e1–84.e7.
- ↑ 1. Souissi S, Le Mer Y, Metge F, Portmann A, Baudouin C, Labbé A, Hamard P. An update on continuous-wave cyclophotocoagulation (CW-CPC) and micropulse transscleral laser treatment (MP-TLT) for adult and paediatric refractory glaucoma. Acta Ophthalmol. 2021 Aug;99(5):e621-e653. doi: 10.1111/aos.14661. Epub 2020 Nov 22.
- ↑ 1. Bai A, Sharma A, Chiang MY. Proliferative Vitreoretinopathy Following Transscleral Diode Cyclophotocoagulation. J Glaucoma. 2023 Jun 1;32(6):e66-e68. doi: 10.1097/IJG.0000000000002222. Epub 2023 Mar 30.
- ↑ 1. Sesma G, Ahmad K, AlBakri A, Awad A, Malik R. Incidence and outcomes of microbial keratitis after cyclophotocoagulation to treat childhood refractory glaucoma. J AAPOS. 2022 Jun;26(3):124.e1-124.e5. doi: 10.1016/j.jaapos.2022.01.009. Epub 2022 May 4.