Cosmetic Applications of Botulinum Toxin in Oculoplastics
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
History
Botulinum toxin has played several roles throughout history. In the 1700s, the first written accounts in Germany linked it to food poisonings by blood sausages.[1] Later on, during World War 2, scientists attempted to weaponize its paralytic effects for biological warfare.[2] During the late twentieth century, scientists began experimenting with its therapeutic potential in medicine.
Botulinum toxin was first tested in 1973 by an ophthalmologist, Alan Scott, MD, as an experimental treatment for strabismus and was approved in 1989 to treat strabismus, blepharospasm, and hemifacial spasm.[3][4] In 2002, the FDA approved its cosmetic usage to treat glabellar wrinkles, which was the launching point for a multibillion-dollar industry that continues to thrive today.[5][6] Over the years, several companies have utilized differing methods of preparation to develop commercial formulations of botulinum toxin, including Botox (OnabotulinumtoxinA), Dysport (AbobotulinumtoxinA), Xeomin (IncobotulinumtoxinA), Jeuveau (PrabotulinumtoxinA), and Daxxify (DaxibotulinumtoxinA). There are high rates of patient-reported satisfaction with botulinum toxin therapy across disorders, suggesting a high impact on quality of life.[7] In oculoplastics, the most common cosmetic applications of botulinum toxin are for reducing forehead and glabellar wrinkles, periocular winkles, and for eyebrow repositioning.[8][9][10]
Pharmacology
The gram-positive, anaerobic bacillus Clostridium botulinum produces seven different antigenic serotypes (A-G).[11] These toxins are 150-kd polypeptides composed of a 100-kd heavy chain and 50-kd light chain linked together by disulfide bonds.[12] They act by binding irreversibly to presynaptic cholinergic receptors, upon which these molecules are endocytosed.[13][14] They then cleave and deactivate SNARE proteins, which are normally responsible for the fusion of acetylcholine vesicles with the cell membrane.[15] By blocking the release of presynaptic acetylcholine vesicles, botulinum toxin thereby causes muscle paralysis.[16]
The most common commercial formulations of botulinum toxin derive from toxin serotype A. The onset of action of botulinum toxin A is 24-72 hours and the maximum effect will occur within 7-14 days.[11] The duration of action on striated muscle is 3-4 months.
The different commercial formulations of botulinum toxin differ by their method of preparation and their potency.[8][17] Their preparations differ by the nontoxic accessory proteins attached to the 150-kd active toxin A.[18] Botox, Dysport, and Xeomin have been used commercially for a longer period of time and are consequently more researched in the literature. They have been used to treat glabellar and forehead lines, eyebrow repositioning, and periorbital wrinkles, among other uses.[19][20][21][22][23][24][25][26] Jeuveau and Daxxify are more recent on the market and have mainly been used to treat glabellar lines. [27][28][29] There is no significant difference in efficacy or safety profile among the different formulations.[18][30][31] The most recent research trials suggest that Daxxify may be useful for long-lasting treatment with a median duration of efficacy of 6 months, while most other botulinum toxin formulations have a shorter duration of efficacy of 3 months.[28]
| Trade name (proprietary name) | Manufacturer | Onset of action | Length of therapeutic effect | Units per vial | Molecular weight (kDa) | Approximate conversion ratio of product to Botox | FDA approved uses |
|---|---|---|---|---|---|---|---|
| Botox (OnabotulinumtoxinA) | Allergan Inc., Irvine, CA, USA | 3-5 days | 3-6 months | 50 or 100 | 900 | 1:1 | Glabellar lines (2002), periorbital wrinkles (2013), forehead lines (2017) |
| Dysport (AbobotulinumtoxinA) | Slough Berkshire, UK | 24 hours | 3-6 months | 300 or 500 | 500-900 | 3:1 | Glabellar lines (2009) |
| Xeomin (IncobotulinumtoxinA) | Merz Pharmaceuticals, Frankfurt, Germany | 5-7 days | 3-6 months | 100 | 150 | 1:1 | Glabellar lines (2011) |
| Jeuveau (PrabotulinumtoxinA) | Daewoong Pharmaceutical, Seoul, South Korea | 3-5 days | 3-6 months | 50 or 100 | 900 | 1:1 | Glabellar lines (2019) |
| Daxxify (DaxibotulinumtoxinA) | Revance Therapeutics, Inc., Newark, CA | 1-2 days | 6-9 months | 50 or 100 | 150 | 2:1 | Glabellar lines (2022) |
Clinical applications
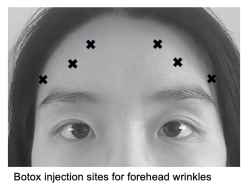
Forehead lines
Forehead wrinkles are formed by repeated upward pulling of the frontal muscle, especially of the medial fibers. Botox can be applied to the lines that form when the patient engages in maximal contraction of the frontal muscle, at typically 6-8 injection sites with a dosage of 10-15 U Botox or 20-30 U Dysport divided among all sites.[10][27] Before addressing forehead wrinkles, it is important to assess the presence of brow ptosis or blepharoptosis to prevent exacerbation.
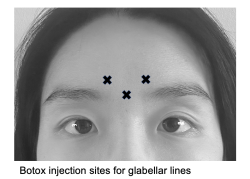
Glabellar lines
Glabellar lines are frown lines between the eyebrows that form due to repeated downward pulling of the procerus and depressor. The horizontal contraction of the corrugator supercilii muscles created the rhytids colloquially known as “11’s”. While Botox and Dysport have been traditionally used to treat glabellar lines, Jeuveau and Daxxify are more recent drugs with comparable efficacy and safety profiles.[29][31] Female patients are commonly treated with 20 U Botox or 50 U Dysport divided among 5 injection sites, while men may need a higher dosage of at least 40 U Botox and 60 U Dysport.[32][33][34]
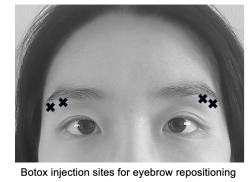
Eyebrow repositioning
While brow position and shape preferences vary across cultures and facial shapes, in the United States, an arched eyebrow with the highest point over the lateral canthus is generally viewed to be aesthetically pleasing.[35][36][37] With aging, the lateral brow tends to be the first to experience descent, while the medial brow may descend with gravity or rise due to compensatory upward pull of the frontalis muscle.[38] Botox injected below the lateral brow at the orbicularis oculi can weaken the depressor muscle and provide a temporary and small lateral brow lift for patients.[39][40] Typically a dosage of 10-15 U Botox or 30-40 U Dysport is divided among three sites inferior to the lateral third of the brow (see image).[10] Although it is unpredictable how much lateral brow elevation will be achieved with an injection, it has been reported that this procedure can cause as much as a brow elevation of 5 mm above the lateral canthus.[39] Since it is imperative to prevent diffusion of the toxin to adjacent orbital muscles, a conservative dose should be given with follow-up injections if desired.
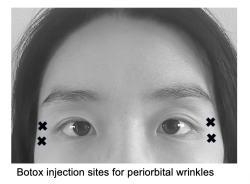
Periorbital wrinkles
Periorbital wrinkles result from repeated radial movement of the orbicularis oculi muscle, resulting in wrinkles along the lateral corner of the eye (known colloquially as “crows feet”) . Neurotoxin of 12 U Botox, 30 U Dysport, or 12 U Xeomin divided among 3 injection sites 1 cm from the lateral orbital rim can weaken the sphincter function of this muscle and soften wrinkle lines.[10][41][42] Care must be taken to avoid the zygomaticus major and levator labii superioris muscles, which fuse with the deep surface of the orbicularis oculi muscle, as this may result in lip ptosis.[43]
Complications and side effects
One issue that may arise with repeated or escalating doses of botulinum toxin treatment is that patients may develop resistance to the toxin. It is reported, however, that fewer than 2% of patients undergoing botulinum toxin therapy will develop neutralizing antibodies over the long-term.[44] To avoid immunologic resistance, patients should be injected with the lowest dose possible for achieving clinical effect and avoid reinjection within one month.[11]
Cosmetic usage of botulinum toxin has a low incidence of adverse effects and most complications are temporary.[45] General side effects may include nausea, fatigue, flu-like symptoms, rashes, and headache.[46] Side effects localized to the injection site may include muscle weakness, pain, edema, ptosis, ecchymosis, headache, and hypesthesia.[45][47] Side effects may also include diplopia, ectropion, drooping lower eyelid, and asymmetric smile.[45][48]
The following conditions are among the exclusion criteria for botulinum toxin usage: pregnancy or breastfeeding, neuromuscular conditions such as myasthenia-gravis and Lambert-Eaton syndrome, and medications that may interact with botulinum toxin such as aminoglycosides, penicillamine, quinine, and calcium channel blockers.[45] While Botox has not been associated with teratogenic events so far, it has not been definitely established as safe during pregnancy.[49][50]
References
- ↑ França K, Kumar A, Fioranelli M, Lotti T, Tirant M, Roccia MG. The history of Botulinum toxin: from poison to beauty. Wien Med Wochenschr. 2017;167(Suppl 1):46-48. doi:10.1007/s10354-017-0553-7
- ↑ Lamanna C, McElroy OE, Eklund HW. The purification and crystallization of Clostridium botulinum type A toxin. Science. 1946;103(2681):613.
- ↑ Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12(12):924-927.
- ↑ Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007;52(1):13-31. doi:10.1016/j.survophthal.2006.10.003
- ↑ Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol. 1992;18(1):17-21. doi:10.1111/j.1524-4725.1992.tb03295.x
- ↑ Niamtu J 3rd. Complications in fillers and Botox. Oral Maxillofac Surg Clin North Am. 2009;21(1):13-v. doi:10.1016/j.coms.2008.11.001
- ↑ Jankovic J, Esquenazi A, Fehlings D, Freitag F, Lang AM, Naumann M. Evidence-based review of patient-reported outcomes with botulinum toxin type A. Clin Neuropharmacol. 2004;27(5):234-244. doi:10.1097/01.wnf.0000145508.84389.87
- ↑ 8.0 8.1 Başar E, Arıcı C. Use of Botulinum Neurotoxin in Ophthalmology. Turk J Ophthalmol. 2016 Dec;46(6):282-290. doi: 10.4274/tjo.57701. Epub 2016 Dec 1. PMID: 28050326; PMCID: PMC5177786.
- ↑ Naik, M. N. DNB; Soparkar, Charles N. S. MD, PhD; Murthy, R. FRCS; Honavar, S. G. MD. Botulinum Toxin in Ophthalmic Plastic Surgery. Indian Journal of Ophthalmology 53(4):p 279-288, Oct–Dec 2005. | DOI: 10.4103/0301-4738.18915
- ↑ 10.0 10.1 10.2 10.3 Stephan S, Wang TD. Botulinum toxin: clinical techniques, applications, and complications. Facial Plast Surg. 2011;27(6):529-539. doi:10.1055/s-0031-1298786
- ↑ 11.0 11.1 11.2 Nigam PK, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55(1):8-14. doi:10.4103/0019-5154.60343
- ↑ Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43(2 Pt 1):249-259. doi:10.1067/mjd.2000.105567
- ↑ Binz T, Rummel A. Cell entry strategy of clostridial neurotoxins. J Neurochem. 2009;109(6):1584-1595. doi:10.1111/j.1471-4159.2009.06093.x
- ↑ Dolly JO, Black J, Williams RS, Melling J. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984;307(5950):457-460. doi:10.1038/307457a0
- ↑ Kao I, Drachman DB, Price DL. Botulinum toxin: mechanism of presynaptic blockade. Science. 1976;193(4259):1256-1258. doi:10.1126/science.785600
- ↑ Choudhury S, Baker MR, Chatterjee S, Kumar H. Botulinum Toxin: An Update on Pharmacology and Newer Products in Development. Toxins (Basel). 2021;13(1):58. Published 2021 Jan 14. doi:10.3390/toxins13010058
- ↑ Bach K, Simman R. The Multispecialty Toxin: A Literature Review of Botulinum Toxin. Plast Reconstr Surg Glob Open. 2022;10(4):e4228. Published 2022 Apr 6. doi:10.1097/GOX.0000000000004228
- ↑ 18.0 18.1 Scaglione F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins (Basel). 2016;8(3):65. Published 2016 Mar 4. doi:10.3390/toxins8030065
- ↑ Roggenkämper P, Jost WH, Bihari K, Comes G, Grafe S; NT 201 Blepharospasm Study Team. Efficacy and safety of a new Botulinum Toxin Type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312. doi:10.1007/s00702-005-0323-3
- ↑ Jost WH, Kohl A, Brinkmann S, Comes G. Efficacy and tolerability of a botulinum toxin type A free of complexing proteins (NT 201) compared with commercially available botulinum toxin type A (BOTOX) in healthy volunteers. J Neural Transm (Vienna). 2005;112(7):905-913. doi:10.1007/s00702-004-0234-8
- ↑ Jost WH, Kohl A, Brinkmann S, Comes G. Efficacy and tolerability of a botulinum toxin type A free of complexing proteins (NT 201) compared with commercially available botulinum toxin type A (BOTOX) in healthy volunteers. J Neural Transm (Vienna). 2005;112(7):905-913. doi:10.1007/s00702-004-0234-8
- ↑ Sampaio C, Ferreira JJ, Simões F, et al. DYSBOT: a single-blind, randomized parallel study to determine whether any differences can be detected in the efficacy and tolerability of two formulations of botulinum toxin type A--Dysport and Botox--assuming a ratio of 4:1. Mov Disord. 1997;12(6):1013-1018. doi:10.1002/mds.870120627
- ↑ Ranoux D, Gury C, Fondarai J, Mas JL, Zuber M. Respective potencies of Botox and Dysport: a double blind, randomised, crossover study in cervical dystonia. J Neurol Neurosurg Psychiatry. 2002;72(4):459-462. doi:10.1136/jnnp.72.4.459
- ↑ Wohlfarth K, Sycha T, Ranoux D, Naver H, Caird D. Dose equivalence of two commercial preparations of botulinum neurotoxin type A: time for a reassessment?. Curr Med Res Opin. 2009;25(7):1573-1584. doi:10.1185/03007990903028203
- ↑ Kollewe K, Mohammadi B, Köhler S, Pickenbrock H, Dengler R, Dressler D. Blepharospasm: long-term treatment with either Botox®, Xeomin® or Dysport®. J Neural Transm (Vienna). 2015;122(3):427-431. doi:10.1007/s00702-014-1278-z
- ↑ Lorenc ZP, Kenkel JM, Fagien S, et al. A review of AbobotulinumtoxinA (Dysport). Aesthet Surg J. 2013;33(1 Suppl):13S-7S. doi:10.1177/1090820X12474632
- ↑ 27.0 27.1 Beer KR, Shamban AT, Avelar RL, Gross JE, Jonker A. Efficacy and Safety of PrabotulinumtoxinA for the Treatment of Glabellar Lines in Adult Subjects: Results From 2 Identical Phase III Studies. Dermatol Surg. 2019;45(11):1381-1393. doi:10.1097/DSS.0000000000001903
- ↑ 28.0 28.1 Solish N, Carruthers J, Kaufman J, Rubio RG, Gross TM, Gallagher CJ. Overview of DaxibotulinumtoxinA for Injection: A Novel Formulation of Botulinum Toxin Type A. Drugs. 2021;81(18):2091-2101. doi:10.1007/s40265-021-01631-w
- ↑ 29.0 29.1 Solish N, Ascher B, Avelar RL, et al. PrabotulinumtoxinA vs OnabotulinumtoxinA for the Treatment of Adult Males With Moderate to Severe Glabellar Lines: Post-hoc Analyses of the Phase III Clinical Study Data. Aesthet Surg J. 2022;42(12):1460-1469. doi:10.1093/asj/sjac210
- ↑ Cheon HI, Jung N, Won CH, Kim BJ, Lee YW. Efficacy and Safety of Prabotulinumtoxin A and Onabotulinumtoxin A for Crow's Feet: A Phase 3, Multicenter, Randomized, Double-Blind, Split-Face Study. Dermatol Surg. 2019;45(12):1610-1619. doi:10.1097/DSS.0000000000001920
- ↑ 31.0 31.1 Carruthers J, Solish N, Humphrey S, et al. Injectable DaxibotulinumtoxinA for the Treatment of Glabellar Lines: A Phase 2, Randomized, Dose-Ranging, Double-Blind, Multicenter Comparison With OnabotulinumtoxinA and Placebo. Dermatol Surg. 2017;43(11):1321-1331. doi:10.1097/DSS.0000000000001206
- ↑ Gadarowski MB, Ghamrawi RI, Taylor SL, Feldman SR. PrabotulinumtoxinA-xvfs for the Treatment of Moderate-to-Severe Glabellar Lines. Ann Pharmacother. 2021;55(3):354-361. doi:10.1177/1060028020943527
- ↑ Ascher B, Zakine B, Kestemont P, Baspeyras M, Bougara A, Santini J. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines [published correction appears in J Am Acad Dermatol. 2005 Jan;52(1):156]. J Am Acad Dermatol. 2004;51(2):223-233. doi:10.1016/j.jaad.2003.11.084
- ↑ Kane MAC, Brandt F, Rohrich RJ, et al. Evaluation of variable-dose treatment with a new U.S. Botulinum Toxin Type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619-1629. doi:10.1097/PRS.0b013e3181b5641b
- ↑ Sclafani AP, Jung M. Desired position, shape, and dynamic range of the normal adult eyebrow. Arch Facial Plast Surg. 2010;12(2):123-127. doi:10.1001/archfacial.2010.17
- ↑ Hwang SJ, Kim H, Hwang K, Kim SK, Kim YS, Kim YS. Ideal or Young-Looking Brow Height and Arch Shape Preferred by Koreans. J Craniofac Surg. 2015;26(5):e412-e416. doi:10.1097/SCS.0000000000001877
- ↑ Patil SB, Kale SM, Jaiswal S, Khare N, Math M. Effect of aging on the shape and position of the eyebrow in an Indian population. Aesthetic Plast Surg. 2011;35(6):1031-1035. doi:10.1007/s00266-011-9728-6
- ↑ Asaad M, Kelarji AB, Jawhar CS, et al. Eyebrow Height Changes with Aging: A Systematic Review and Meta-analysis. Plast Reconstr Surg Glob Open. 2019;7(9):e2433. Published 2019 Sep 30. doi:10.1097/GOX.0000000000002433
- ↑ 39.0 39.1 Ahn MS, Catten M, Maas CS. Temporal brow lift using botulinum toxin A. Plast Reconstr Surg. 2000;105(3):1129-1139. doi:10.1097/00006534-200003000-00046
- ↑ Uygur S, Eryilmaz T, Bulam H, Yavuzer R, Latifoglu O. The quantitative effect of botulinum toxin A over brow height [published correction appears in J Craniofac Surg. 2013 Sep;24(24):1827]. J Craniofac Surg. 2013;24(4):1285-1287. doi:10.1097/SCS.0b013e318292c80c
- ↑ Ascher B, Rzany BJ, Grover R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow's feet: double-blind, placebo-controlled, dose-ranging study. Dermatol Surg. 2009;35(10):1478-1486. doi:10.1111/j.1524-4725.2009.01261.x
- ↑ Prager W, Wissmüller E, Kollhorst B, Böer A, Zschocke I. Botulinumtoxin Typ A im Halbseitenvergleich. Wirksamkeit und Verträglichkeit bei der Behandlung von Krähenfüßen [Treatment of crow's feet with two different botulinum toxin type A preparations in split-face technique]. Hautarzt. 2011;62(5):375-379. doi:10.1007/s00105-011-2148-3
- ↑ Matarasso SL, Matarasso A. Treatment guidelines for botulinum toxin type A for the periocular region and a report on partial upper lip ptosis following injections to the lateral canthal rhytids. Plast Reconstr Surg. 2001;108(1):208-217. doi:10.1097/00006534-200107000-00033
- ↑ Mohammadi B, Buhr N, Bigalke H, Krampfl K, Dengler R, Kollewe K. A long-term follow-up of botulinum toxin A in cervical dystonia. Neurol Res. 2009;31(5):463-466. doi:10.1179/174313209X405137
- ↑ 45.0 45.1 45.2 45.3 Klein AW. Complications and adverse reactions with the use of botulinum toxin. Semin Cutan Med Surg. 2001;20(2):109-120. doi:10.1053/sder.2001.25964
- ↑ Bakheit AM. The possible adverse effects of intramuscular botulinum toxin injections and their management. Curr Drug Saf. 2006;1(3):271-279. doi:10.2174/157488606777934431
- ↑ Witmanowski H, Błochowiak K. The whole truth about botulinum toxin - a review. Postepy Dermatol Alergol. 2020;37(6):853-861. doi:10.5114/ada.2019.82795
- ↑ Ahsanuddin S, Roy S, Nasser W, Povolotskiy R, Paskhover B. Adverse Events Associated With Botox as Reported in a Food and Drug Administration Database.
- ↑ Tan M, Kim E, Koren G, Bozzo P. Botulinum toxin type A in pregnancy. Can Fam Physician. 2013;59(11):1183-1184.
- ↑ Li W, Tang M. Application of botulinum toxin in pregnancy and its impact on female reproductive health. Expert Opin Drug Saf. 2020;19(1):83-91. doi:10.1080/14740338.2020.1707803