Corneal Keloids
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Introduction

A corneal keloid is a benign pearly, gray-white epicorneal lesion (Figure 1) that results from abnormal proliferation of fibrous tissue and accumulation of disorganized collagen fibrils and glycoprotein, with characteristic hyperplasia of the corneal epithelium and disruption of Bowman's layer.[2] It can develop after ocular trauma or infection, or can arise congenitally and/or idiopathically. Bilateral cases of corneal keloids are typically associated with congenital disorders including Lowe Syndrome and Rubenstein-Taybi Syndrome.[2][3] Unlike cutaneous keloids, corneal keloids are rare and less than 100 cases have been documented in the literature since it was first described in 1865.[4][5][6] Nonetheless, they can cause significant ocular morbidity, including decreased vision and poor cosmesis, and thus warrant attention. The following will discuss corneal keloids in detail, including associated risk factors, presentation, differential diagnosis, diagnosis, pathogenesis, and treatment.
Clinical Presentation
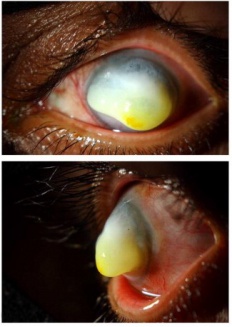
While reported ages of presentation range from 2 months to 72 years, the majority of corneal keloids occur in the first 3 decades of life.[8][9][10] The typical clinical presentation is a slowly-enlarging, solitary, raised epicorneal lesion with a gray-white glistening appearance (Figure 1) associated with painless progressive vision loss over months to years, a result of an abnormal corneal reparative process (see Pathogenesis). If sufficiently large, the lesion can interfere with closure of the eyelids and cause exposure keratopathy (Figure 2).[7][11]
Corneal keloids have no established link to cutaneous keloids or cutaneous hypertrophic scar formation except in Rubenstein-Taybi Syndrome.[2][12] Accordingly, 3 studies on long-term follow-up after LASIK of patients with history of dermatologic keloids report no incidence of corneal keloids or hypertrophic scarring (total eyes n = 34). [13][14][15] In fact, there has been only one case reported in which subepithelial corneal scarring (not keloid) developed following refractive surgery in a patient with propensity for cutaneous keloid formation.[16] Furthermore, unlike cutaneous keloids where there is higher incidence in African Americans and Asians relative to Caucasians, no such association has been delineated with corneal keloids.[12] There is, however, higher incidence of corneal keloids in males.[10][17]
Most patients who develop corneal keloids have a remote history of corneal trauma (ex. fingernail injury, ocular radiation[18][19]), corneal disease (ex. bullous keratopathy[6]), recurrent keratouveitis[6] or ocular surgery involving corneal incision (ex. cataract surgery, vitrectomy[17][20]). Interestingly, several patients with remote history of eyelid surgery (no corneal incision) have also developed corneal keloids, suggesting that exposure keratopathy may precede keloid formation in absence of penetrating corneal wound in susceptible individuals.[17] In addition to the above, systemic diseases, notably rubeola and Lowe Syndrome (oculocerebrorenal syndrome), have also been associated with corneal keloid development.[2][3] And while extremely rare, corneal keloids have also arisen in complete absence of ocular history or systemic diseases--yet some researchers propose a possible etiology of unrecognized corneal infection that could trigger an immune response resulting in keloid formation in few of these cases.[4][5][9][9][21] Thus, absence of remote ocular trauma cannot exclude corneal keloid as a diagnosis for a glistening, gray-white protuberant epicorneal mass.
Diagnosis

The diagnosis of corneal keloid is achieved through both clinical and histopathological examinations, the latter of which establishes the characteristic presence of collagen, fibroblasts and myofibroblasts, and is the gold standard for diagnosis.[8][9][10][1] Oftentimes, clinical suspicion for keloid is sufficient to warrant diagnostic and therapeutic surgical removal (see Treatment), and subsequent histopathologic analyses with immunohistochemical staining under light microscopy can serve to confirm the diagnosis.[9] Ultrastructural analyses with use of electron microscopy have also been employed in case studies of keloids, but are not required for diagnosis.[9][1][22][23]
Clinical evaluation
Apart from a thorough history-taking, clinical evaluation of a suspected corneal keloid include visual acuity assessment and slit-lamp biomicroscopy. Ultrasound biomicroscopic echography can also add valuable information, such as the depth of lesion involvement and concurrent abnormalities of surrounding tissues.[7] Depending on the location, size, and age of the corneal keloid, visual acuity can vary from preserved to hand motion or even light perception. On slit-lamp biomicroscopy, typical features visualized include a smooth, pearly-white elevated opacity with well-demarcated margins, and the lesion may or may not be vascularized (Figure 3).[4][6][7][12][19][20][1][22] Oftentimes, the keloid spans over much of the cornea, rendering applanation tonometry, anterior chamber examination, and dilated fundoscopy impossible.[4][7][12] Of note, development of the keloid itself does not alter structures posterior to the cornea, but the associated ocular trauma that predisposes a keloid, such as a perforating injury or radiation, can render associated abnormalities in the anterior chamber depth, lens, iris, and fundus otherwise unaffected by keloid growth itself.[6][7][19][20] In cases where the keloid obscures in-depth view on slit-lamp, ultrasonography can be advantageous to assess involvement of surrounding structures (ex. uveal tissue prolapse, extensive synechiae, angle involvement) and to plan for excision, especially in cases of extensive ocular trauma.[7][20] Regarding the keloid itself, ultrasound biomicroscopic echography and/or B-scan ultrasonography typically depict a hyperdense lesion well-separated from the surrounding normal corneal tissue and an unremarkable posterior segment, barring prior injury or glaucoma.[4][6][11][12][20][22][24]
Histopathological and ultrastructural evaluation

Light Microscopy: As previously mentioned, histopathological examination typically follows excision of suspected corneal keloids for confirmation of diagnosis. The excised corneal keloid, when stained with hematoxylin-eosin and periodic acid-Schiff and viewed under light microscopy, demonstrates the following characteristic features (Figure 4):[4][6][9][7][12]
- Location: keloid mass is visualized between an irregular epithelium and a disrupted Bowman’s layer
- Epithelium: marked irregularity with hyperplasia (sometimes hypoplasia), acanthosis, parakeratosis, and an edematous cellular disorganization of the basal layer.
- Bowman’s layer: often fragmented, disrupted, or completely absent
- Stroma: edematous, scarred, with loss of normal lamellar pattern and increased thickness; increased number of keratocytes and prominence of spindle-shaped fibroblasts and myofibroblasts; occasionally, inflammatory cells are seen; stromal neovascularization is often visualized; trichrome stain can highlight the erratic collagen bundles and whorls associated with fibrosis in the stroma; positive immunohistochemical stains with anti-actin and anti-vimentin, confirming presence of myofibroblasts and fibroblasts.
- Descemet’s membrane: can be irregularly thick, or normal
- Endothelium: intact, normal
 Figure 5 Electron micrograph of randomly-oriented collagen fibrils in the lesion. (x18000 mag). (Reproduced from the British Journal of Ophthalmology, Risco et al [1]with permission from BMJ Publishing Group Ltd.)
Figure 5 Electron micrograph of randomly-oriented collagen fibrils in the lesion. (x18000 mag). (Reproduced from the British Journal of Ophthalmology, Risco et al [1]with permission from BMJ Publishing Group Ltd.)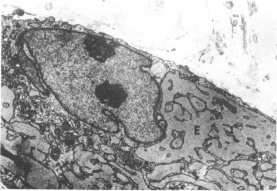 Figure 6 Electron micrograph of the intracellular structures of an activated fibroblast. Note the two nuclei and markedly dilated rough endoplasmic reticulum surrounding the nucleus. (Reproduced from the British Journal of Ophthalmology, Risco et al [1] with permission from BMJ Publishing Group Ltd.)
Figure 6 Electron micrograph of the intracellular structures of an activated fibroblast. Note the two nuclei and markedly dilated rough endoplasmic reticulum surrounding the nucleus. (Reproduced from the British Journal of Ophthalmology, Risco et al [1] with permission from BMJ Publishing Group Ltd.)
It is important to note that the histopathologic features vary with stage of the keloid. Notably, in the early stages, there is a predominance of stromal fibroblasts/myofibroblasts associated with new collagen deposits and neovascularization. In the late stage, known as the “hyaline” stage, collagen fibers become compact, with rare fibroblasts and shriveled vessels.[12][18][20]
Electron Microscopy: As with examination under light microscopy, examination under electron microscopy also reveals epithelial hyperplasia and acanthosis, disrupted basal layer, and thickened stroma with irregularly oriented collagen fibrils (Figure 5).[9][1][22][23] Macrophage-like cells can be found in the stroma.[1] In addition to the above, electron microscopy allows visualization of intracellular structures of the activated fibroblasts, including: fibrillar cytoplasm, irregular nuclei with compact chromatin, abundant cytoplasm with prominent rough endoplasmic reticulum, and increased amounts of mitochondria, glycogen, and granules (Figure 6).[9][18][1][22] Again, findings on ultrastructural studies can differ based on stage of keloid, and in the later stages, activated fibroblasts are rarer.
Differential Diagnoses
In cases where a corneal keloid arises after known corneal insult, a hypertrophic corneal scar is a reasonable differential diagnosis.[6][22] The characteristic differences are that keloids extend beyond the initial wound and can develop months to years after the initial insult. In contrast, hypertrophic scars tend to appear immediately after a trauma and do not grow significantly beyond the boundaries of the initial wound. A corneal inclusion cyst presenting as an avascular white pearly mass , but can also arise after ocular trauma and is another possible differential diagnosis.[12] Histopathological examination showing acellular eosinophilic material can differentiate inclusion cyst from keloid (see Histopathological and ultrastructural evaluation).[12]
Other differential diagnoses for an elevated gray-white corneal lesion (regardless of history of corneal insult) are corneal myxoma, Salzmann’s nodular degeneration, Peter’s anomaly, congenital hereditary endothelial dystrophy, limbal dermoid*, anterior staphyloma, squamous cell carcinoma, juvenile xanthogranuloma, fibrous histiocytoma, corneal ulcer, and metabolic disease[11][17][19][1] *One case report established corneal keloid as the diagnosis for a recurrent mass following dermoid excision.[25]
Pathogenesis
The exact pathogenesis of corneal keloids remains obscure with debate as to whether keloids originate from iris stroma or corneal stroma, although there is growing evidence and argument for the latter. Experts in the early 1900s argued for iris origin, based on sole observations of keloids that formed after corneal perforation with prolapse of iris tissue, and subsequent keloid neovascularization that seemed to arise from iris stroma.[7][26][27] However, modern data point toward a mechanism of corneal stroma overgrowth with transformation of keratocytes (corneal stromal cells) into fibroblasts and myofibroblasts (cells involved in wound formation) under the influence of inflammatory markers during the healing process. This is further supported by cases of keloids arising without corneal perforation, or with corneal injury but without iris involvement, and where keloid vessels originate from the peripheral cornea.[9][27][28] Mejia and colleagues proposed that cytokines released by injured epithelium interfere with normal corneal healing, triggering fibrovascular overgrowth.[9] In agreement, Dhooge et al speculate the involvement of high levels of the cytokine BMP4 (bone morphogenetic protein 4) could affect signaling of TGF-B (transforming growth factor beta), a cytokine involved in corneal stroma differentiation and wound contraction, thus resulting in overstimulation of fibroblastic growth.[29] Furthermore, in separate studies of rabbit and mouse corneal keratocytes, Jester et al and Reneker et al demonstrated that TGFB1 induced keratocyte transformation into myofibroblasts with increased smooth-muscle actin expression.[30][31] As an aside, in Lowe Syndrome, where the incidence of corneal keloid is relatively higher, an underlying systemic abnormality is implicated in keloid formation, but like that of keloids in general, the exact mechanism remains obscure.[27]


Management
Treatment
There is often a watch and wait period (spanning months to years) for suspected corneal lesions that are small and asymptomatic.[6][9][18][22] Bourcier et al assert that surgical management should be reserved for visually significant or symptomatic lesions, as there is risk of keloid exacerbation when inflicting a corneal wound during surgical removal.[22] Unfortunately, initial medical management alone with corticosteroids has been tried previously without lesion regression, or cessation of keloid growth.[5] Thus, early management in cases of suspected corneal keloid is close monitoring until the visual axis is heavily compromised, after which surgery is then considered.[6][9][18][22] Depending on depth and size of keloid, as well as the presence of concurrent structural abnormalities (ex. synechiae), surgical options vary and include local excision with superficial lamellar keratectomy (SLK),[6][9][12][17][18] phototherapeutic keratectomy (PTK), deep anterior lamellar keratoplasty (DALK)[32], penetrating keratoplasty (PK)[4][9][21], sclerokeratoplasty[24], keratoprosthesis[4], and enucleation.[19] Many of the above options yield variable results in terms of recurrence rates and visual outcomes in different reports in the literature. There has been recurrence reported after excision[17][23], SLK[4][9][17][18], PTK[4], and PK[4]. It is hypothesized that recurrence in SLK (as compared to DALK) is likely due to residual stromal tissue containing activated fibroblasts; yet, such hypothesis only partially explains keloid recurrence, as there is one report of keloid recurrence after PK.[4] It has also been hypothesized that use of a temporary amniotic membrane (to cover bare stroma), which has anti-fibroblastic and anti-inflammatory properties, may help prevent recurrence in SLK. However, Lee et al reported recurrence in after SLK with amniotic membrane in 4 patients even with use of adjunctive Mitomycin C in 2 of the patients (Figure 7).[17] In contrast, Fukuda et al reported successful use of tranilast eyedrops, a mast cell stabilizer known to inhibit collagen synthesis in fibroblasts, as adjuvant therapy to SLK, with no keloid recurrence observed in the patient 12 years from intervention.[5] Finally, scarring around sutures have occurred in some cases, without interference of the visual axis (Figure 8).[4][9][21]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Risco JM, Huaman A, Antonios SR. A case of corneal keloid: clinical, surgical, pathological, and ultrastructural characteristics. The British Journal of Ophthalmology 1994;78(7):568-71
- ↑ Jump up to: 2.0 2.1 2.2 2.3 Bukowiecki A, Hos D, Cursiefen C, Eming SA. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. International Journal of Molecular Sciences 2017;18(6):1257 doi: 10.3390/ijms18061257[published Online First: Epub Date]|.
- ↑ Jump up to: 3.0 3.1 Esquenazi S, Eustis HS, Bazan HE, Leon A, He J. Corneal keloid in Lowe syndrome. J Pediatr Ophthalmol Strabismus 2005;42(5):308-10
- ↑ Jump up to: 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 Bakhtiari P, Agarwal DR, Fernandez AA, et al. Corneal Keloid: Report of Natural History and Outcome of Surgical Management in Two Cases. Cornea 2013;32(12):1621-24 doi: 10.1097/ICO.0b013e3182a73a10[published Online First: Epub Date]|.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Fukuda K, Chikama T, Takahashi M, Nishida T. Long-term follow-up after lamellar keratoplasty in a patient with bilateral idiopathic corneal keloid. Cornea 2011;30(12):1491-4 doi: 10.1097/ICO.0b013e31822018f2[published Online First: Epub Date]|.
- ↑ Jump up to: 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 Gupta J, Gantyala SP, Kashyap S, Tandon R. Diagnosis, Management, and Histopathological Characteristics of Corneal Keloid: A Case Series and Literature Review. Asia Pac J Ophthalmol (Phila) 2016;5(5):354-9 doi: 10.1097/apo.0000000000000154[published Online First: Epub Date]|.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Alkatan HM, Al-Arfaj KM, Hantera M, Al-Kharashi S. Healed corneal ulcer with keloid formation. Saudi Journal of Ophthalmology 2012;26(2):245-48 doi: 10.1016/j.sjopt.2011.10.005[published Online First: Epub Date]|.
- ↑ Jump up to: 8.0 8.1 Holbach LM, Font RL, Shivitz IA, Jones DB. Bilateral keloid-like myofibroblastic proliferations of the cornea in children. Ophthalmology 1990;97(9):1188-93
- ↑ Jump up to: 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 9.15 9.16 Mejía LF, Acosta C, Santamaría JP. Clinical, Surgical, and Histopathologic Characteristics of Corneal Keloid. Cornea 2001;20(4):421-24
- ↑ Jump up to: 10.0 10.1 10.2 Vanathi M, Panda A, Kai S, Sen S. Corneal keloid. Ocul Surf 2008;6(4):186-97
- ↑ Jump up to: 11.0 11.1 11.2 Mendez EA, Daza MT. Sclerokeratoplasty in a case of corneal keloid. Cornea 1991;10(2):183-4
- ↑ Jump up to: 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 Chawla B, Agarwal A, Kashyap S, Tandon R. Diagnosis and management of corneal keloid. Clin Exp Ophthalmol 2007;35(9):855-7 doi: 10.1111/j.1442-9071.2007.01608.x[published Online First: Epub Date]|.
- ↑ Lee JY, Youm DJ, Choi CY. Conventional Epi-LASIK and lamellar epithelial debridement in myopic patients with dermatologic keloids. Korean J Ophthalmol 2011;25(3):206-9 doi: 10.3341/kjo.2011.25.3.206[published Online First: Epub Date]|.
- ↑ Artola A, Gala A, Belda JI, et al. LASIK in myopic patients with dermatological keloids. J Refract Surg 2006;22(5):505-8
- ↑ Cobo-Soriano R, Beltran J, Baviera J. LASIK outcomes in patients with underlying systemic contraindications: a preliminary study. Ophthalmology 2006;113(7):1118.e1-8 doi: 10.1016/j.ophtha.2006.02.023[published Online First: Epub Date]|.
- ↑ Girgis R, Morris DS, Kotagiri A, Ramaesh K. Bilateral corneal scarring after LASIK and PRK in a patient with propensity to keloid scar formation. Eye 2005;21(1):96-97
- ↑ Jump up to: 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 17.8 17.9 Lee HK, Choi HJ, Kim MK, Wee WR, Oh JY. Corneal keloid: four case reports of clinicopathological features and surgical outcome. BMC Ophthalmology 2016;16:198 doi: 10.1186/s12886-016-0372-4[published Online First: Epub Date]|.
- ↑ Jump up to: 18.0 18.1 18.2 18.3 18.4 18.5 18.6 Shoukrey NM, Tabbara KF. Ultrastructural study of a corneal keloid. Eye (London, England) 1993;7 ( Pt 3):379-87 doi: 10.1038/eye.1993.76[published Online First: Epub Date]|.
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 Jung JJ, Wojno TH, Grossniklaus HE. Giant Corneal Keloid: Case Report and Review of the Literature. Cornea 2010;29(12):1455-58 doi: 10.1097/ICO.0b013e3181d83858[published Online First: Epub Date]|.
- ↑ Jump up to: 20.0 20.1 20.2 20.3 20.4 20.5 Singh A, Sen S, Vanathi M, Tandon R. Corneal keloid with cystoid cicatrix:post–small-incision cataract surgery. Canadian Journal of Ophthalmology / Journal Canadien d'Ophtalmologie 2017;52(3):e93-e95 doi: http://dx.doi.org/10.1016/j.jcjo.2016.11.013[published Online First: Epub Date]|.
- ↑ Jump up to: 21.0 21.1 21.2 21.3 21.4 Song J-S, Kwon S, Shyn K-H. A Case of Congenital Corneal Keloid. Korean J Ophthalmol 2005;19(2):156-59
- ↑ Jump up to: 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 22.8 Bourcier T, Baudrimont M, Boutboul S, Thomas F, Borderie V, Laroche L. Corneal keloid: clinical, ultrasonographic, and ultrastructural characteristics. J Cataract Refract Surg 2004;30(4):921-4 doi: 10.1016/j.jcrs.2003.08.025[published Online First: Epub Date]|.
- ↑ Jump up to: 23.0 23.1 23.2 Rao SK, Fan DS, Pang CP, et al. Bilateral congenital corneal keloids and anterior segment mesenchymal dysgenesis in a case of Rubinstein-Taybi syndrome. Cornea 2002;21(1):126-30
- ↑ Jump up to: 24.0 24.1 Mendez EA, Daza MT. Sclerokeratoplasty in a case of corneal keloid. Cornea 1991;10(2):183-4
- ↑ Gaviria JG, Johnson DA, Scribbick F, 3rd. Corneal keloid mimicking a recurrent limbal dermoid. J Pediatr Ophthalmol Strabismus 2005;42(3):189-90
- ↑ Farkas TG, Znajda JP. Keloid of the cornea. Am J Ophthalmol 1968;66(2):319-23
- ↑ Jump up to: 27.0 27.1 27.2 McElvanney AM, Adhikary HP. Corneal keloid: Aetiology and management in Lowe's syndrome. Eye 1995;9(3):375-76
- ↑ O'Grady RB, Kirk HQ. Corneal keloids. Am J Ophthalmol 1972;73(2):206-13
- ↑ Dhooge MR, Idema AJ. Fibrodysplasia ossificans progressiva and corneal keloid. Cornea 2002;21(7):725-9
- ↑ Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Progress in retinal and eye research 1999;18(3):311-56
- ↑ Reneker L, Bloch A, Xie L, Overbeek P, Ash J. Induction of Corneal Myofibroblasts by Lens-derived Transforming Growth Factor β1 (TGFβ1): A Transgenic Mouse Model, 2009.
- ↑ Ashar JN, Pahuja S, Ramappa M, Vaddavalli PK, Chaurasia S, Garg P. Deep anterior lamellar keratoplasty in children. Am J Ophthalmol 2013;155(3):570-74.e1 doi: 10.1016/j.ajo.2012.09.029[published Online First: Epub Date]|.

