Cornea Transplantation-Induced Glaucoma: A Review of Glaucoma Secondary to PKP, DMEK, and DSAEK Procedures
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Glaucoma is a serious complication that follows keratoplasty procedures, causing significant morbidity[1]. Cornea transplantation usually induces increased intraocular pressure (IOP), which can accelerate the rate of corneal endothelial cell loss and graft failure[2]. In fact, two leading causes of graft failure post-keratoplasty procedures are graft rejection and secondary glaucoma development[3]. Patients with pre-operative glaucoma were observed to experience approximately twice to triple as many graft rejections as those without pre-existing glaucoma[4]. History of pre-existing glaucoma predisposes eyes to risk of IOP spikes post-keratoplasty. High IOP may also have far-reaching consequences leading to optic nerve damage and irreversible vision loss. This review aims at highlighting the incidence, mechanisms, and management of glaucoma following different corneal transplant procedures. We will focus mainly on the commonly performed procedures like penetrating keratoplasty (PKP), Descemet's Membrane Endothelial Keratoplasty (DMEK), and Descemet's Stripping Automated Endothelial Keratoplasty (DSAEK).
Background
PKP involves resecting the host cornea and replacing it with a full-thickness donor graft[5]. As it involves replacement of all corneal layers, patients with conditions such as considerable stromal scarring, keratoconus, and bullous keratopathy benefit the most from the procedure. The incidence of glaucoma following PKP is roughly 9 to 50%, and ranges from 10 to 31% in the early post-operative period and from 18 to 35% in the late post-operative period[6][1]. Further, herpes virus infection, trauma, and repeated corneal transplantation were shown to carry an increased risk for secondary glaucoma development post-PKP[2].
DMEK has been widely adopted, since it involves the replacement of only the endothelium and Descemet’s membrane [1]. It is a well recognizable alternative to PKP for diseases that involve corneal endothelial dysfunction. An advantage of DMEK is that it avoids creating stroma-to-stroma interface[5]. Such an interface can often be irregular, which potentially limits vision post-operatively. Common indications for DMEK include Fuch’s dystrophy, bullous keratopathy and graft failure. According to Maier et al., 15.4% of post-DMEK patients develop ocular hypertension within 24 hours after the procedure. Patients undergoing DMEK procedure in combination to cataract surgery and intraocular lens (IOL) implantation tend to have even higher incidence of IOP spikes post-operatively[7].
Similar to DMEK, DSAEK procedures have been continuously increasing, replacing many of the PKP surgeries in cases that can be managed with selective replacement of diseased layers. From 2005 to 2008, the number of endothelial transplants increased from 1,429 to 17,468 cases[8]. PKP cases dwindled from 45,821 to 32,524 cases during the same time period. DSAEK involves transplanting the donor’s endothelium, Descemet’s membrane, and stroma. Being a lamellar endothelial keratoplasty procedure like DMEK, DSAEK has similar indications. In comparison to PKP, DSAEK offers faster recovery, less astigmatism development, and suture-related complications[5]. Yet, similar to PKP and DMEK, DSAEK shows considerable rates of glaucoma development post-operatively. According to one study, the incidence rates of IOP spikes post-DSAEK are 35% if the patient has no history of glaucoma, 45% with prior history, and 43% with pre-existing glaucoma-related surgery[9].
Etiology and Pathophysiology
There are different mechanisms that contribute to keratoplasty-induced glaucoma. It is important to consider such etiologies with respect to the time period they tend to present at, early or late post-operatively, for proper treatment. Early presentation tends to present within the first week(s) after surgery while late manifestation tends to occur in several weeks or months[10]. Pre-existing glaucoma predisposes to increased IOP post-keratoplasty, and can become the culprit early or late following surgery[11].
a. Penetrating Keratoplasty:
Early-Onset Glaucoma:
1) Viscoelastic Agent:
Viscoelastic material is applied in PKP procedures in order to ensure maintaining a physical depth between the posterior transplanted cornea and underlying structures including the iris and the lens[12]. This prevents corneal endothelial damage that can arise from mechanical injury. As much as it is essential to maintain corneal graft’s survival, viscoelastic substance is associated with increased incidence of post-PKP glaucoma[13]. Viscoelastics are generally divided into ones with high cohesiveness and others with high dispersiveness; highly cohesive materials are synthesized from a larger number of molecular chains, thus acquire a higher molecular weight and tend to be more readily removed after surgery[14]. Dispersive materials, contrarily, have low molecular weight and share a relatively lower tendency for self-adherence. Cohesive agents include Healon GV, Healon 5 (Advanced Medical Optics [AMO], Santa Ana, CA); Amvisc Plus (Bausch & Lomb, Rochester, NY); as well as Provisc (Alcon, Ft Worth, TX). Dispersive agents include Vitrax (AMO) and Viscoat (Alcon).
The viscoelastics’ high viscosity can cause trabecular meshwork (TM) obstruction, thus hindering aqueous humor outflow from the anterior chamber (AC)[15]. A study conducted by Hozler et al. showed a direct association between increased viscoelastic substance’s viscosity and increased IOP, but there was no statistical significance[14].
2) Suturing Technique and Transplant Size:
In a study by Zimmerman et al., researchers speculated the phenomenon of disproportionate early glaucoma development post-PKP between phakic and aphakic patient populations[16]. The iridocorneal angle is demarcated anteriorly by the Schwalbe’s line (termination of Descemet’s membrane) and posteriorly by the ciliary body (where zonules attach). The aphakic eye lacks adequate posterior support as it lacks the lens-zonule attachment. Moreover, deep or midstromal corneal sutures involved in keratoplasty can produce a posterior wound gape[17]. The Descemet’s membrane, in turn, is weakened and may retract posteriorly towards the iridocorneal angle, undermining the anterior support. Altering support areas might lead to TM collapse, impeding outflow. Whereas phakic eyes suffer the weakening of only the anterior support, aphakic eyes are vulnerable to weakening anteriorly and posteriorly. This accounts for the observation that aphakic eyes more readily develop post-PKP glaucoma.
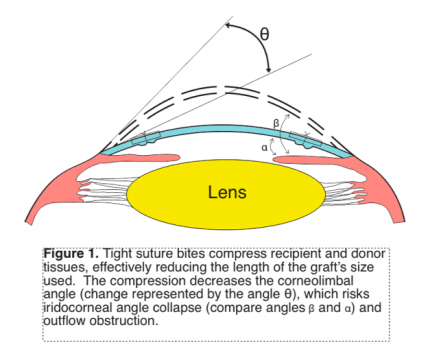
Suturing the graft and host’s tissues tightly compresses the recipient and donor’s sides against each other. This practically reduces the length of the donor button and decreases the corneal curvature; corneal tissue that extends from the limbus has to swing towards the corneal center to unite with the graft (Figure 1). This lessens the corneolimbal angle, which risks compression of the iridocorneal angle. A combination of same sized donor button as the recipient’s bed, tight sutures, and long bites further compresses tissues and causes crowding that can compromise the TM.
3) Inflammation:
PKP, and surgeries in general, induce tissue deformation, which promotes a state of inflammation. This leads to disruption of the blood-aqueous barrier (BAB)[18]. As a result, inflammatory cells and normal serum components flow into the aqueous humor in large amounts. They can clump up and become entrapped, impeding the outflow system. Further, fibrin-rich exudates can form adhesions connecting the iris to the trabecular meshwork (peripheral anterior synechiae, [PAS]) and/or to the lens (posterior synechiae). This can cause outflow obstruction and rapidly develop into pupillary block, causing secondary angle-closure glaucoma[11].
Late-Onset Glaucoma:
1) Corticosteroid-Induced Glaucoma:
Post-PKP use of corticosteroid is essential to prevent endothelial rejection and to maintain graft survival. However, steroids are postulated to inhibit phagocytic properties in the TM. This promotes the accumulation of cellular debris and obstructs outflow, which induces glaucoma[19]. Another potential mechanism through which steroids administration can induce glaucoma is through water retention[20][21]. Corticosteroids can stabilize lysosomal membranes, thus reducing lysosomal hyaluronidase. The subsequent accumulation of mucopolysaccharides leads to edema and narrowing of the TM.
Furthermore, a study of dexamethasone’s behavior in the human TM cells has shown that intravenously administered steroids binds preferentially to the nuclei of the outflow channel’s cells[22][23]. Steroids induce cellular growth and proliferation as well as increase the rate of deposition of extracellular matrix material. Such an exaggerated anabolic effect can factor into mechanically obstructing outflow, thus inducing IOP spike.
Corticosteroids have also been shown to exert their glaucomatous effects by influencing specific genes. The myocilin (formerly known as TM-inducible glucocorticoid response [TIGR]) gene is one of the most studied chromosomal regions associated with glaucoma and is found to be upregulated in response to glucocorticoids; its 55 kDa protein product is highly expressed in the TM cells post-treatment with dexamethasone[24]. Although the exact mechanism underlying myocilin’s effects are not well-understood, experiments show that the dose needed to increase IOP is similar to that needed to cause myocilin’s expression. Moreover, the time period until steroid-induced IOP spike manifest correlates with that of myosin gene’s increased expression. Mutations in the gene, found in chromosome 1, have also been implicated in a variety of juvenile and adult onset primary open angle glaucoma (POAG)[25].
Approximately 21% of glaucoma development secondary to PKP is a result of corticosteroid usage[2]. It is noteworthy to mention that the frequency of steroid-induced rise in IOP post-PKP is more pronounced in patients with keratoconus and Fuchs dystrophy, 73% and 60.3% respectively[3].
Not all corticosteroids exert their detrimental effects with equal potency. Mindel et al. reported a significant difference among medrysone, fluorometholone, and dexamethasone phosphate with respect to their tendencies to induce IOP spike[26]. Over a six-week period, dexamethasone is expected to increase IOP twice as much as fluoromethasone, while eight times as much as medrysone. Moreover, difluprednate 0.05% is a relatively newer synthetic steroidal agent that is more potent than prednisolone acetate 1%. It is associated with 21% IOP rise (comparable to other agents) but has been shown to increase the risk of epitheliopathy[27]. In general, considerations should be made to maintain graft survival post-PKP with the least clinically feasible dose and frequency to reduce the risk of steroid-induced IOP spike.
2) Persistent Inflammation:
In case post-PKP inflammation persists for long periods, adhesions may form from the periphery of the iris to the TM—PAS[11]. This occurrence is theorized to occur more often with an atrophic iris. PAS may lead to iridocorneal angle compression and induce IOP rise.
Vajpayee et al. observed that the greater the oversize margin of the donor bed, the less likely that PAS would form post-operatively[28]. Patients with a history of iridocorneal adhesions undergoing PKP are at an increased risk of developing PAS after surgery, despite adequate suturing and synechiolysis. It is postulated that patients with pre-operative iridocorneal adhesions have the lens-iris diaphragm positioned more anteriorly. The floppy iris continues to assume this abnormal position causing the anterior chamber to be shallow, thus more susceptible to synechiae formation post-PKP. Vajpayee et al. showed that 30% of patients with grafts oversized by 0.25 mm to 0.5 mm showed PAS manifestations after six to twelve months of follow-up. Contrarily, no patient with grafts oversized by 1 mm developed PAS post-PKP. This is likely because the 1 mm oversized graft was able to maintain optimal depth of the AC, thus reducing the risk of iridocorneal adhesions.
b. Descemet's Membrane Endothelial Keratoplasty:
Early-Onset Glaucoma:
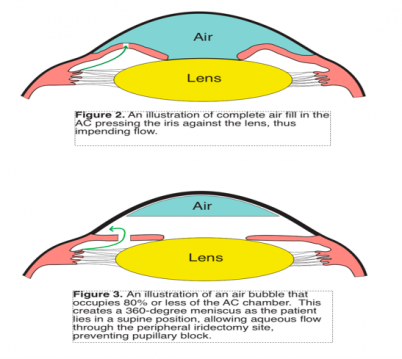
An important mechanism for early glaucoma development in the immediate post-DMEK period is air bubble-induced angle closure. Melles et al. established a method to fixate the donor tissue to the host’s bed without sutures; he suggested introducing an air bubble in the AC to aid in holding the graft tightly to the host’s posterior stroma[29]. IOP rise almost always occurs until 2 hours following DMEK; however, persistently high IOP is estimated to take place in 12.5% of patients[30] Larger air bubbles minimize the risk of graft detachment post-DMEK. Peripheral iridectomy should permeate flow of the aqueous humor when pupillary pathway is blocked in case the iris presses strongly against the lens. However, when the air bubble completely occupies the AC’s volume, there remains no volume for the aqueous flow into the AC through the patent peripheral iridectomy; thus, pressure builds up in the posterior chamber, as trapped fluid pushes against the iris, creating a posterior pupillary block (Figure 2).
Patients sitting in an upright position immediately post-DMEK may develop simultaneous anterior and posterior pupillary blocks. Peripheral iridectomy is traditionally performed in the 12-o’clock position . If an air bubble is injected such that it only partially fills up the AC, and the patient sits in the upright position, air will float upwards being less dense. This can render the iridectomy site dysfunctional as the air bubble takes space over it, and aqueous fluid may potentially overwhelm the inferior iris, displacing it forward. Both of these effects push the iris posteriorly (superiorly) and anteriorly (inferiorly), leading to angle closure.
Injected air bubble may also spread to the posterior chamber, pushing the iris anteriorly. This compresses the iridocorneal angle and obstructs aqueous flow.
Patients undergoing simultaneous DMEK and cataract surgery with IOL implantation show a greater likelihood of developing post-operative ocular hypertension or glaucoma than those receiving only DMEK. This could possibly be explained by multiple mechanisms including increased inflammation, altered angle anatomy, and the fact that the pseudophakic eyes exhibit a flexible IOL-iris diaphragm, which allows for more air to be injected into the AC. Greater air volume pumped, in turn, is more conducive to pupillary block development.
Similar to PKP-induced glaucoma, the cause of IOP spike in the early post-DMEK period can be from left-over viscoelastic material[31]. Viscoelastic material can be injected between the iris and the graft to prevent air bubble escape from the AC. Retained viscoelastic substance can induce IOP spike, as explained beforehand in post-PKP glaucoma.
Late-Onset Glaucoma:
The etiologies of observed increased IOP in the late post-operative period are not different from those seen in late-onset glaucoma for post-PKP patients. Blockage by inflammatory cells, PAS formation, and steroid use are common causes[31].
It is noteworthy to mention that steroid-induced glaucoma post-DMEK is not as common as that post-PKP or post-DSAEK. This is likely due to the short steroid regime post-DMEK that is usually sufficient to control inflammation and prevent IOP elevation.
c. Descemet's Stripping Automated Endothelial Keratoplasty:
The etiologies of early or late glaucoma development post-DASEK are not very different from those encountered post-PKP or post-DMEK[30]. Similar to the DMEK procedure, DSAEK procedure involves the injection of an air bubble into the AC to adhere the graft to the host tissue. As such, the most common cause of early post-operative glaucoma development is pupillary block, similar to the mechanism of early IOP spike presentation in DMEK. Air in the AC can lead to pupillary block, while air that has intruded posterior to the iris may cause forward iris rotation and angle closure. In one study, pupillary block post-DSAEK was estimated to occur at a rate of 13%.
Prolonged Steroid use is a major cause of late-onset glaucoma in post-DSAEK patients. According to one study, 18.6% of post-DSAEK patients developed glaucoma secondary to steroid administration. Inflammatory glaucoma is still a possible etiology in post-DSAEK patients, but it tends to occur much less frequently than in post-PKP patients.
Diagnostic Workup
Pre-operative corneal disease presents a challenge to accurately assess IOP and visual field to determine a baseline for comparison in the post-operative period[32]. It is also difficult to measure IOP post-keratoplasty given changes in corneal thickness and the development of irregular astigmatism[33]. Additionally, epithelial edema and scar tissue can result in falsely reduced and elevated readings, respectively. As such, Goldmann Applanation Tonometer (GAT) readings can be imprecise as GAT is sensitive to corneal biomechanical variations.
The Dynamic Contour Tonometer (DCT) serves as an alternate tool to measure IOP since it functions more independently from some mechanical factors that affect applanation tonometry[32]. The DCT apparatus involves a tonometer tip that is supplied with a surface contour. When the contour is placed against the corneal surface, the appositional force applied changes the corneal shape to resemble that of the contour. The distribution of the forces at the interphase counterbalances force distribution produced by the IOP. In that manner, a centrally located sensor at the tonometer’s tip is capable of measuring eye pressure transcorneally, mostly independent of corneal irregularities[34][35].
Ocular Response Analyzer (ORA) is another device that can assess IOP accounting for some corneal biomechanics[36]. It functions by generating a rapid air pulse and analyzing the consequential corneal indentation. The pulse induces corneal applanation, slight indentation beyond applanation point, and reversion back to original position. A light emitter is directed at the corneal surface and a detector monitors the peak light intensity reflected off. Peak light intensities coincide with applanation, which occurs twice as the corneal surface moves inward and back outwards. The mean between the two output applanation air pulse pressures determines the IOP. In that way ORA can be useful for IOP assessment after corneal transplantation, which may alter corneal mechanical properties.
According to Kandarakis at al., although differences between DCT and GAT IOP measurements post-PKP are not significant, DCT tends to be more adapt[37]. GAT is affected by corneal astigmatism and has been shown to underestimate pressure reading post-PKP by multiple studies[38][39]; Kandarakis at al. reported an average IOP measurement by DCT to be 16.6 (SD 2.8) mmHg, while that obtained by GAT to be 15.1 (SD 3.6) mmHg.
ORA measurements have shown a considerably wide difference from GAT’s[40]. Chou et al. showed a mean of 6.29 mmHg higher reading of IOP with ORA than with GAT. The difference might be attributed to the linear calibration coefficient that converts the average of the two applanation pressures to an IOP that is Goldmann-related. Biomechanical corneal changes post-PKP may, thus, necessitate an adjusted coefficient to be more reliable.
Changes in central corneal thickness (CCT) is not as significant post-DSAEK as in post-PKP; with an average post-operative CCT of 621 µm, post-DSAEK CCT is close to that of the upper limit of a normal cornea (596 µm)[41]. For the minimal corneal alteration, GAT is considered a safely reliable method of IOP monitoring post-operatively. Bochmann et al. reported a post-DSAEK IOP of 19.1 (± 6.5) mmHg and 20.9 (± 5.9) mmHg by GAT and DCT, respectively. Considering the well-studied average difference between GAT and DCT IOP readings (about 1.7 mmHg) in normal corneas, the relative maintenance of such a difference (1.8 mmHg) in post-DSAEK corneas suggests the validity of using DCT or GAT post-operatively[42].
Compared to DSAEK corneas, and to a greater extent PKP corneas, post-DMEK corneas can maintain nearly normal anatomy. As they do not involve additional stroma, DMEK grafts do not cause significant corneal curvature changes and almost negligibly alter CCT[43]. Moreover, after surgery, the eye is not edematous. Therefore, GAT may still be utilized for IOP measurement post-DMEK. Similar to GAT, noncontact pneumatic tonometry (NCT) and iCare are other IOP measuring devices that might be influenced by CCT. In a study by Maier et al., NCT and iCare correlated with CCT post-operatively, while GAT and DCT did not[43]. All four methods, however, uncovered a pathologic IOP that needed treatment, and are valid devices for routine examination post-DMEK.
Disc evaluation that may include Optical Coherence Tomography or photographs should be taken on the first examination post-keratoplasty to serve as a baseline; imaging should be repeated as necessary or annually in case of glaucoma to detect any development or progression of glaucomatous optic neuropathy[6]. Diagnosis of post-keratoplasty ocular hypertension or glaucoma is established by IOP measurements early post-operatively, and by IOP measurement, visual field analysis, and optic disc changes late post-operatively.
PAS formation causing secondary angle closure is an important etiology of raised IOP post-keratoplasty in patients with totally opaque grafts[44]. Hence, ultrasound biomicroscopy (UBM) can be an important tool for determining appropriate sites for glaucoma drainage device (GDD) placement and glaucoma filtering surgeries for this patient population. UBM is also especially useful after corneal transplantation as it can assess iridocorneal adhesions, AC depth, angle width, and corneal thickness[6].
Management
Since PKP has been the leading means of cornea transplantation before the relatively recent advent of DMEK and DSAEK, the bulk of scientific literature on post-keratoplasty glaucoma management has been studied on PKP patients. Nevertheless, the medical and surgical options available for managing IOP spikes in all three procedure types tend to be similar.
Pre-Operatively:
Operating on an eye with uncontrolled IOP can risk rapid decompensation post-keratoplasty. Hence, pre-existent glaucoma needs to be managed prior to surgery[32]. If high IOP is not adequately controlled with medical treatment, surgical intervention should be sought prior to corneal transplantation.
Intra-Operatively:
Graft sizes that are 0.5-1 mm longer than the host bed have been associated with lower risks of glaucoma development post-PKP[16]. This is likely because slightly longer host beds do not cause significant corneolimbal alteration discussed in the mechanisms section. Moreover, oversized grafts provide optimal AC depth, which reduces the risk of PAS formation and resultant IOP spike[28].
The suturing technique implemented is also of critical importance. It has been shown that short and equal sutures between the host and the graft bring forth favorable consequences due to less distortions of tissues and derangement of angle architecture[32]. This is likely due to relatively minimal crowding of graft and host tissues, which decreases the tendency of iridocorneal angle collapse. Further, tight graft sutures prevent incarceration of the iris at the host-graft junction.
Immediately after corneal transplantation, topical steroid administration is essential to control inflammation[32]. This reduces the risk of posterior and peripheral anterior synechiae formation, which can potentially develop pupillary block with IOP elevation. If given for long periods, it is imperative to monitor corticosteroid dosage given in order to reduce the risk of developing secondary steroid induced glaucoma. It is also common practice to use mydriatics in the early post-keratoplasty period to prevent pupillary block. Of note, Price et al. studied IOP elevations with the use of prednisolone acetate 1% compared to fluorometholone 0.1% (weaker agent) in DMEK patients. They concluded that significantly more eyes treated with prednisolone acetate surpassed the IOP elevation threshold of the study. This is despite the fact that the Fluorometholone-treated group involved more eyes with pre-operative glaucoma diagnosis[45].
Leftover viscoelastic material increases the risk of TM blockage. Hence, it is crucial to remove the material as completely as possible at the conclusion of the transplantation procedure[32].
To prevent pupillary block secondary to total air filling in DMEK and DSAEK, it is recommended to fill the AC incompletely with 80% or less volume fill with air (Figure 3)[30]. This circumvents pupillary block by disallowing air to push against the pupil, obstructing aqueous flow; air injected will rest on top of a 360-degree meniscus of aqueous fluid, which permeates aqueous fluid’s passage through the site of the peripheral iridectomy . Further, IOP may spike from pupil block when sitting in an upright position post-DMEK or DSAEK as discussed previously. This can be prevented by creating an inferior peripheral iridectomy, rather than one in the 12-o’clock position. That way aqueous flow is less likely to be hindered by the injected air bubble that will float superiorly as the patient assumes an upright position.
Post-Operatively:
Medical Treatment:
The initial post-operative management of keratoplasty-induced glaucoma is through topical medications[6]. Beta adrenergic blocking agents, adrenergic agonist, and topical carbonic anhydrase inhibitors lower IOP by decreasing aqueous humor production. Generally topical agents have a rapid onset, and are appropriate medications for long-term effect. While adverse effects are largely tolerable, topical agents have been associated with punctate epithelial keratopathy and corneal anesthesia that may affect the graft’s epithelium[32]. Brimonidine tartarate (0.15% or 0.2%) is typically used three times daily if prescribed as a monotherapy, or twice if in combination with other agents[6]. Newer RhoKinase inhibitors may be used but are not well studied in this patient population.
Oral carbonic anhydrase inhibitors use is typically only implemented early post-operatively, but if later usage is needed, topical CAIs are more preferred. Topical CAIs do not carry the same significant side effects of the systemic version (metabolic acidosis, paresthesia, gastrointestinal upset, kidney stones, among others)[32]. CAIs have been shown to interfere with carbonic anhydrase function in the corneal epithelium and endothelium causing irritative symptoms and risking edema and graft decompensation, although very rare[46]. Therefore, patients on CAIs should be monitored for those adverse effects.
As opposed to beta-blockers, topical CAI and alpha-agonists, prostaglandin analogs are used once a day, and thus are a more adequate choice for chronic post-cornea transplantation glaucoma. These agents are given once daily and are associated with fewer systemic effects. There are no direct associations found in the literature between prostaglandin analogs’ use and graft rejections in PKP, DSAEK, or DMEK. However, careful and regular monitoring for side effects is important, as their use in general has been shown to increase the risk of developing cystoid macular edema[47]. Some studies have also shown recurrence of herpetic keratitis in patients with a history of the disease, and so prostaglandin analogs should be prescribed with caution for patients in this population who undergo cornea transplant procedures[48]. Despite the possible complications, there is no hesitation to use them in patients with cornea transplant-induced glaucoma, particularly when surgery is the only alternative. Latanoprostene bunod, a nitric oxide donating molecule, is a relatively newer agent for IOP lowering that may have utility in this setting although there are no studies in this patient population.
Laser Treatment:
Patients with keratoplasty-induced glaucoma that is not controlled with medications can be treated with selective laser trabeculoplasty (SLT). SLT has been largely implemented due to its relatively few complications, and comparable efficacy to argon laser trabeculoplasty (ALT). It is also repeatable. SLT is notable for avoiding collateral thermal damage to treated tissues[49]. It targets the pigmented cells in the TM, and typically functions at a power setting of 0.4-1.2 mJ. The device delivers 400 μm laser spots in 3 ns. Adding to the advantages of SLT treatment, it is not associated with PAS formation[50]. It is important to note that although SLT has been shown to control elevated IOP secondary to keratoplasty procedure, IOP needs to be monitored as most literature is in open angle glaucoma and post cornea transplant eyes may have a varying long term effect.
Nd:YAG laser iridotomy can be utilized in post-keratoplasty patients with pupillary blockage. However, the peripheral cornea may not be transparent enough, which may pose visibility challenges to performing the procedure[6]. In those instances a more central location for the iridotomy may be necessary.
Surgical Treatment:
Trabeculectomy:
Trabeculectomy is one of the surgical interventions to consider for post-keratoplasty glaucoma treatment that is progressive and refractory to medical therapy. The impact of trabeculectomy in the treatment of post-PKP and post-DSAEK glaucoma is studied, but its efficacy for post-DMEK glaucoma management is relatively lacking in the literature. Elhofi et al. explored the efficacy of trabeculectomy compared to Ahmed glaucoma valve (AGV) in the management of post-PKP glaucoma[51]. They concluded that although AGV effectively controls IOP, trabeculectomy shows longer periods of graft survival; graft survival one year post-operatively were 75% and 85% for AGV implant and trabeculectomy, respectively.
Trabeculectomy seems to be more effective at controlling IOP in post-DSAEK eyes than post-PKP ones. One theory accounting for the higher success is that the AC stability is better maintained in post-DSAEK eyes[52]. PKP requires suturing that can compromise the AC angle, promoting PAS formation and secondary glaucoma. A year after trabeculectomy shows significantly different average IOP of 10.6 mmHg and 14.6 mmHg for post-DSAEK and post-PKP patients, respectively.
In patients undergoing trabeculectomy, it is crucial to consider the complications that can arise from 5-fluorouracil (5-FU) administration. As many as 50% of patients given 5-FU for several days after trabeculectomy are found to experience corneal epithelial side effects and graft cloudiness, likely secondary to the toxic effects of 5-FU on the corneal epithelium[53]. For this reason, mitomycin C (MMC) is currently routinely used instead. Trabeculectomy with MMC has been shown to have approximately three times the success rate of glaucoma control as standard trabeculectomy[54]. It is typically applied one to four minutes (0.2-0.4 mg/ml) intra-operatively[6]. MMC is not without complications, though, as it is associated with increased incidence of bleb leaks, infection, hypotony, choroidal detachment and macular edema.
Challenges of subconjunctival filtering surgery in eyes with prior surgery include scar formation and the need for additional surgical interventions. This is likely due to the severe subconjunctival fibrosis secondary to prior interventions, and PAS formation[55]. Both factors can affect the long-term outcomes after trabeculectomy.
Glaucoma Drainage Devices (GDDs):
GDDs are generally also widely used to control IOP if medical therapy is not sufficient in progressive disease[56]. Different types of GDDs can be used: valved (Ahmed valve; Krupin implant) and valveless (Molteno implant; Baerveldt implant). Complication rates following implantation of a GDD, like corneal decompensation, and overall success rates appear to be comparable among various types of devices.
As previously mentioned, GDD is often associated with higher rates of graft failure than trabeculectomy, and thus, is often considered when trabeculectomy fails. Yet, GDDs can reasonably control refractory IOP in PKP-induced glaucoma. A study of the literature shows that IOP control after GDD implantations in PKP patients ranges from 51% to 96% during 13 to 74 months of follow up[57][58][59][60][61]. Sherwood et al. showed that Molteno implants post-PKP maintained an IOP of 18 mmHg or less in 96% of patients at 22 months mark post GDD implant[62]. Alvarenga et al. reported slightly less impressive results with IOP control in 74% and 63.1% of patients at one and two years, respectively.
Graft survival after GDD implantation for refractory PKP-induced glaucoma is widely variable. Multiple studies in the literature reported values between 49% and 90%. Alvarenga et al. reported survival rates of 58.25% and 25.8% at one and two years, respectively[63]. Further, the efficacy of GDD implantation simultaneously with PKP, before keratoplasty, or after keratoplasty with respect to graft survival is uncertain. Kwon et al. observed that patients who had GDD first experienced 3.8 and 4.7 as much graft failure as the simultaneous and PKP-first patients, respectively[64]. On the other hand, Rapuano et al. reported 44% graft failure in the PKP first group, compared to 29% and 31% for the simultaneous and GDD first groups, respectively[61].
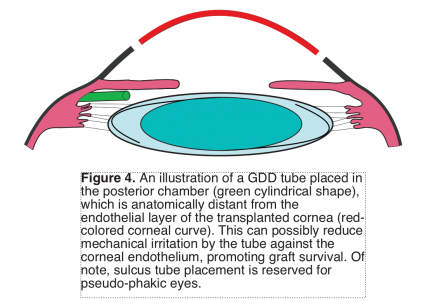
Data on the efficacy of GDD for post-DMAEK and post-DSAEK patients are relatively more lacking. However, challenges that could be confronted with post-DSAEK patient involve the nature of the thick edge of the corneal graft; this can potentially block the tube lumen of the GDD device[13]. Thus, it is preferable to insert the tube into the posterior chamber to avoid impingement against the thick corneal edge.
Some of the disadvantages of installing GDDs are the associated greater graft failure rates than trabeculectomy[64]. Two mechanisms account for the greater failure incidences with GDDs in post- keratoplasty patients: immunological and mechanical[32]. The inserted tube is thought to promote a path for inflammatory cells’ flow from the subconjunctival space into the AC. Moreover, the inserted tube may, due to its proximity, mechanically damage the graft’s endothelium, increasing the rate of endothelial failure. Persistent topical steroid use for months/years post-keratoplasty can help in suppressing inflammation. Further, implanting the tube in the posterior chamber can potentially reduce the risk to the corneal endothelium and has been shown to provide similar success rates (Figure 4).
Cyclodestructive Procedures:
Cyclophotocoagulation (CPC) has been an adopted intervention for post-keratoplasty glaucoma. It is generally considered a last resort option for refractory cases who have undergone trabeculectomy and/or GDD implantation. Beiran et al. and Ocakoglu et al. reported successful IOP control of 70% and 72%, respectively, after one year of CPC treatment in post-PKP patients[65]. Beiran et al. further showed 63% success after five years of the CPC intervention.
Subramaniam et al. studied the outcomes of micropulse transscleral cyclophotocoagulation (TSCPC) for IOP control in post-keratoplasty eyes including post-PKP, post-DSAEK, and post-DMEK eyes[66]. They reported the mean IOP after 12 months to have been 15 mmHg ± 5. 71% of the eyes showed IOP reduction of 20 mmHg or more. Lee et al. reported an almost identical observation with 72% of post-keratoplasty patients exhibiting at least 20 mmHg IOP decrease after a year[67]. Aquino et al. attempted to compare the efficacy of continuous CPC versus MicroPulse-CPC (MPCPC), and reported 30% reduction in 75% and 29% of continuous CPC and MPCPC treated patients at 12 months, respectively[68].
One of the complications post-CPC is the intraocular inflammation which can increase the risk of rejection, and the occurrence of hypotony; titration of pulsed TSCPC may reduce this risk. Of note, Subramaniam et al. reported a mean of approximately 10% loss of endothelial cell density post-CPC[66].
References
- ↑ 1.0 1.1 1.2 Haddadin, Ramez I., and James Chodosh. “Corneal Transplantation and Glaucoma.” Seminars in Ophthalmology, vol. 29, no. 5-6, 2014, pp. 380–396., doi:10.3109/08820538.2014.959201.
- ↑ 2.0 2.1 2.2 Baltaziak, Monika, et al. “Glaucoma after Corneal Replacement.” Survey of Ophthalmology, vol. 63, no. 2, 2018, pp. 135–148., doi:10.1016/j.survophthal.2017.09.003.
- ↑ 3.0 3.1 Al-Mahmood, Ammar M., et al. “Glaucoma and Corneal Transplant Procedures.” Journal of Ophthalmology, vol. 2012, 2012, pp. 1–9., doi:10.1155/2012/576394.
- ↑ Janson, Ben J., et al. “Glaucoma-Associated Corneal Endothelial Cell Damage: A Review.” Survey of Ophthalmology, vol. 63, no. 4, 2018, pp. 500–506., doi:10.1016/j.survophthal.2017.11.002.
- ↑ 5.0 5.1 5.2 Boynton, Grace E., and Maria A. Woodward. “Evolving Techniques in Corneal Transplantation.” Current Surgery Reports, vol. 3, no. 2, 2015, doi:10.1007/s40137-014-0079-5.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Dada, Tanuj, et al. “Post-Penetrating Keratoplasty Glaucoma.” Indian Journal of Ophthalmology, vol. 56, no. 4, 2008, p. 269., doi:10.4103/0301-4738.41410.
- ↑ Maier AK, Wolf T, Gundlach E, et al. Intraocular pressure elevation and post-DMEK glaucoma following Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2014;252:1947–1954.
- ↑ Eye Banking Statistical Report. Washington, District of Columbia, USA: EyeBank Association of America; 2007.
- ↑ Banitt, Michael R, and Vikas Chopra. “Descemetʼs Stripping with Automated Endothelial Keratoplasty and Glaucoma.” Current Opinion in Ophthalmology, vol. 21, no. 2, 2010, pp. 144–149., doi:10.1097/icu.0b013e3283360b95.
- ↑ Rumelt, Shimon. “Glaucoma in Cases of Penetrating Keratoplasty, Lamellar Procedures and Keratoprosthesis.” Glaucoma - Basic and Clinical Concepts, 2011, doi:10.5772/23895.
- ↑ 11.0 11.1 11.2 Greenlee, Emily C., and Young H. Kwon. “Graft Failure: III. Glaucoma Escalation after Penetrating Keratoplasty.” International Ophthalmology, vol. 28, no. 3, 2008, pp. 191–207., doi:10.1007/s10792-008-9223-5.
- ↑ Sugar, Alan, and Joel Sugar. “Techniques in Penetrating Keratoplasty.” Cornea, vol. 19, no. 5, 2000, pp. 603–610., doi:10.1097/00003226-200009000-00005.
- ↑ 13.0 13.1 Al-Mahmood, Ammar M., et al. “Glaucoma and Corneal Transplant Procedures.” Journal of Ophthalmology, vol. 2012, 2012, pp. 1–9., doi:10.1155/2012/576394.
- ↑ 14.0 14.1 Holzer, Mike P, et al. “Effect of healon5 and 4 Other Viscoelastic Substances on Intraocular Pressure and Endothelium after Cataract Surgery.” Journal of Cataract & Refractive Surgery, vol. 27, no. 2, 2001, pp. 213–218., doi:10.1016/s0886-3350(00)00568-x.
- ↑ Stamper , Robert, et al. “Effect of Intraocular Aspiration of Sodium Hyaluronate on Postoperative Intraocular Pressure.” Ophthalmic Surgery, Lasers and Imaging Retina, vol. 21, no. 7, 1 July 1990, pp. 486–491., doi:10.3928/1542-8877-19900701-08.
- ↑ 16.0 16.1 Zimmerman, Thom. “Transplant Size and Elevated Intraocular Pressure.” Archives of Ophthalmology, vol. 96, no. 12, 1978, p. 2231., doi:10.1001/archopht.1978.03910060533012.
- ↑ Zimmerman, T. J., et al. “The Effect of Suture Depth on Outflow Facility in Penetrating Keratoplasty.” Archives of Ophthalmology, vol. 96, no. 3, 1978, pp. 505–506., doi:10.1001/archopht.1978.03910050273018.
- ↑ Bodh, Sonama, et al. “Inflammatory Glaucoma.” Oman Journal of Ophthalmology, vol. 4, no. 1, 2011, p. 3., doi:10.4103/0974-620x.77655.
- ↑ Kersey, J P, and D C Broadway. “Corticosteroid-Induced Glaucoma: A Review of the Literature.” Eye, vol. 20, no. 4, 2005, pp. 407–416., doi:10.1038/sj.eye.6701895.
- ↑ Francois J . Corticosteroid glaucoma. Ann Ophthalmol 1977; 9: 1075–1080.
- ↑ Armaly MF . Effect of corticosteroids on intraocular pressure and fluid dynamics: I. The effect of dexamethasone in the normal eye. Arch Ophthalmol 1963; 70: 482–491.
- ↑ Wordinger RJ, Clark AF . Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retina Eye Res 1999; 18: 629–667.
- ↑ Tripathi BJ, Tripathi RC, Swift HH . Hydrocortisone-induced DNA endoreplication in human trabecular cell in vitro. Exp Eye Res 1989; 49: 259–270.
- ↑ Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR . Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem 1998; 273: 6341–6350.
- ↑ Alward WLM, Fingert JH, Coote MA, Johnson T, Lerner SF, Junqua D et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N Engl J Med 1998; 338: 1022–1027.
- ↑ Mindel, J. S., et al. “Comparative Ocular Pressure Elevation by Medrysone, Fluorometholone, and Dexamethasone Phosphate.” Archives of Ophthalmology, vol. 98, no. 9, 1980, pp. 1577–1578., doi:10.1001/archopht.1980.01020040429006.
- ↑ Sorkin, Nir, et al. “Outcomes of Difluprednate Treatment for Corneal Graft Rejection.” Canadian Journal of Ophthalmology, vol. 55, no. 1, 2020, pp. 82–86., doi:10.1016/j.jcjo.2019.07.010.
- ↑ 28.0 28.1 Vajpayee, R. “Oversized Corneal Grafts for Corneal Opacities with Iridocorneal Adhesions.” Ophthalmology, vol. 108, no. 11, 2001, pp. 2026–2028., doi:10.1016/s0161-6420(01)00772-2.
- ↑ Melles GR, Lander F, Nieuwendaal CP. Sutureless, posterior lamellar keratoplasty: a case report of a modified technique. Cornea. 2002;21:325–327.
- ↑ 30.0 30.1 30.2 Röck, Daniel, et al. “Air Bubble–Induced High Intraocular Pressure After Descemet Membrane Endothelial Keratoplasty.” Cornea, vol. 35, no. 8, 2016, pp. 1035–1039., doi:10.1097/ico.0000000000000901.
- ↑ 31.0 31.1 Ang, Marcus, and Chelvin C.a. Sng. “Descemet Membrane Endothelial Keratoplasty and Glaucoma.” Current Opinion in Ophthalmology, vol. 29, no. 2, 2018, pp. 178–184., doi:10.1097/icu.0000000000000454.
- ↑ 32.0 32.1 32.2 32.3 32.4 32.5 32.6 32.7 32.8 Zemba, Mihail, and Alina-Cristina Stamate. “Glaucoma after Penetrating Keratoplasty.” Romanian Journal of Ophthalmology, vol. 61, no. 3, 2017, pp. 159–165., doi:10.22336/rjo.2017.30.
- ↑ Ayyala, Ramesh S. “Penetrating Keratoplasty and Glaucoma.” Survey of Ophthalmology, vol. 45, no. 2, 2000, pp. 91–105., doi:10.1016/s0039-6257(00)00141-7.
- ↑ Sampaolesi, Roberto, et al. “Pascal Tonometer: Dynamic Contour Tonometry.” The Glaucomas, 2013, pp. 153–159., doi:10.1007/978-3-642-35500-4_12.
- ↑ Punjabi, Omar S, et al. “Dynamic Contour Tonometry: Principle and Use.” Clinical & Experimental Ophthalmology, vol. 34, no. 9, 2006, pp. 837–840., doi:10.1111/j.1442-9071.2006.01389.x.
- ↑ Bezerra, Bernardo De Padua Soares, et al. “Intraocular Pressure Measurement after Corneal Transplantation.” Survey of Ophthalmology, vol. 64, no. 5, 2019, pp. 639–646., doi:10.1016/j.survophthal.2019.02.011.
- ↑ Ismail, Andre. “Comparison of Dynamic Contour Tonometry with Goldmann Applanation Tonometry for Measurement of IOP in Patients Following Penetrating Keratoplasty.” Http://Isrctn.org/>, 2012, doi:10.1186/isrctn47431897.
- ↑ Ismail AR, Lamont M, Perera S, et al. Comparison of IOP measurement using GAT and DCT in patients with penetrating keratoplasties. Br J Ophthalmol 2007;91:980–1
- ↑ Ceruti P, Morbio R, Marraffa M, Marchini G. Comparison of dynamic contour tonometry and goldmann applanation tonometry in deep lamellar and penetrating keratoplasties. Am J Ophthalmol 2008;145:215–21
- ↑ Huang, Jinhai, et al. “Comparison of Intraocular Pressure Measurement Using 4 Different Instruments Following Penetrating Keratoplasty.” American Journal of Ophthalmology, vol. 153, no. 3, 2012, p. 580., doi:10.1016/j.ajo.2011.11.028.
- ↑ Bochmann, F, et al. “Comparison of Dynamic Contour Tonometry with Goldmann Applanation Tonometry Following Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK).” Klinische Monatsblätter Für Augenheilkunde, vol. 226, no. 04, 2009, pp. 241–244., doi:10.1055/s-0028-1109242.
- ↑ Kaufmann C, Bachmann L M, Thiel M A. Comparison of dynamic contour tonometry with Goldmann applanation tonometry. Invest Ophthalmol Vis Sci. 2004; 45 3118-3121
- ↑ 43.0 43.1 Maier, Anna-Karina, et al. “Intraocular Pressure Measurements After Descemet Membrane Endothelial Keratoplasty.” Journal of Glaucoma, vol. 26, no. 3, 2017, pp. 258–265., doi:10.1097/ijg.0000000000000593.
- ↑ Dada, Tanuj, et al. “Ultrasound Biomicroscopy in Opaque Grafts With Post-Penetrating Keratoplasty Glaucoma.” Cornea, vol. 27, no. 4, 2008, pp. 402–405., doi:10.1097/ico.0b013e31816373c5.
- ↑ Price, Marianne O., et al. “Randomized Comparison of Topical Prednisolone Acetate 1% Versus Fluorometholone 0.1% in the First Year After Descemet Membrane Endothelial Keratoplasty.” Cornea, vol. 33, no. 9, 2014, pp. 880–886., doi:10.1097/ico.0000000000000206.
- ↑ Konowal, A, et al. “Irreversible Corneal Decompensation in Patients Treated with Topical Dorzolamide.” American Journal of Ophthalmology, vol. 127, no. 4, 1999, pp. 403–406., doi:10.1016/s0002-9394(98)00438-3
- ↑ Kaufman, Herbert E, et al. “Latanoprost Increases the Severity and Recurrence of Herpetic Keratitis in the Rabbit.” American Journal of Ophthalmology, vol. 127, no. 5, 1999, pp. 531–536., doi:10.1016/s0002-9394(99)00089-6.
- ↑ Wand, Martin, et al. “Latanoprost and Herpes Simplex Keratitis.” American Journal of Ophthalmology, vol. 127, no. 5, 1999, pp. 602–604., doi:10.1016/s0002-9394(99)00050-1.
- ↑ Mcalinden, C. “Selective Laser Trabeculoplasty (SLT) vs Other Treatment Modalities for Glaucoma: Systematic Review.” Eye, vol. 28, no. 3, 2013, pp. 249–258., doi:10.1038/eye.2013.267.
- ↑ Nakakura, Shunsuke, et al. “Selective Laser Trabeculoplasty for Glaucoma After Penetrating Keratoplasty.” Optometry and Vision Science, vol. 86, no. 4, 2009, doi:10.1097/opx.0b013e318199d226.
- ↑ Elhofi, Abdelhamid, and Hany Ahmed Helaly. “Graft Survival after Penetrating Keratoplasty in Cases of Trabeculectomy versus Ahmed Valve Implant.” Journal of Ophthalmology, vol. 2018, 2018, pp. 1–6., doi:10.1155/2018/9034964.
- ↑ Boey, Pui Yi, et al. “Outcomes of Trabeculectomy After Descemet Stripping Automated Endothelial Keratoplasty: A Comparison With Penetrating Keratoplasty.” American Journal of Ophthalmology, vol. 153, no. 6, 2012, doi:10.1016/j.ajo.2011.12.014.
- ↑ Gupta, P, et al. “Post Penetrating Keratoplasty Glaucoma – A Review.” Nepalese Journal of Ophthalmology, vol. 6, no. 1, 2014, pp. 80–90., doi:10.3126/nepjoph.v6i1.10776.
- ↑ Ishioka M, Schimezaki J, Jamagami J, Fujishima H, Shimmura S, Tsubota K. Trabeculectomy with mitomycin C for postkeratoplasty glaucoma. Br. J Ophthalmol. 2000; 84(7):714-717
- ↑ Gilvarry, A M E, et al. “The Management of Post-Keratoplasty Glaucoma by Trabeculectomy.” Eye, vol. 3, no. 6, 1989, pp. 713–718., doi:10.1038/eye.1989.110.
- ↑ Kirkness, Colin M. “Penetrating Keratoplasty, Glaucoma and Silicone Drainage Tubing.” Developments in Ophthalmology New Microsurgical Concepts, Posterior and Anterior Segments, pp. 161–165., doi:10.1159/000414385.
- ↑ Ayyala RS (2000) Penetrating keratoplasty and glaucoma. Surv Ophthalmol 45(2):91–105
- ↑ McDonnell PJ, Robin JB, Schanzlin DJ, Minckler D, Baerveldt G, Smith RE et al (1988) Molteno implant for control of glaucoma in eyes after penetrating keratoplasty. Ophthalmology 95(3):364–369
- ↑ Kirkness CM, Ling Y, Rice NS (1988) The use of silicone drainage tubing to control post-keratoplasty glaucoma. Eye 2(Pt 5):583–590
- ↑ Beebe WE, Starita RJ, Fellman RL, Lynn JR, Gelender H (1990) The use of Molteno implant and anterior chamber tube shunt to encircling band for the treatment of glaucoma in keratoplasty patients. Ophthalmology 97(11):1414–1422
- ↑ 61.0 61.1 Rapuano CJ, Schmidt CM, Cohen EJ, Rajpal RK, Raber IM, Katz LJ et al (1995) Results of alloplastic tube shunt procedures before, during, or after penetrating keratoplasty. Cornea 14(1):26–32
- ↑ Sherwood MB, Smith MF, Driebe WT Jr., Stern GA, Beneke JD, Zaum ZS. Drainage tube implants in the treatment of glaucoma following penetrating keratoplasty. Ophthamic Surg. 1993; 24(3):185-189.
- ↑ Alvarenga LS, Mannis MJ, Brandt JD, Lee WB, Schwab IR, Lim MC (2004) The long-term results of keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol 138(2):200–205
- ↑ 64.0 64.1 Kwon, Y. “Long-Term Results of Eyes with Penetrating Keratoplasty and Glaucoma Drainage Tube Implant.” Ophthalmology, vol. 108, no. 2, 2001, pp. 272–278., doi:10.1016/s0161-6420(00)00496-6.
- ↑ Beiran I, Rootman DS, Trope GE, Buys YM (2000) Long-term results of transscleral Nd:YAG cyclophotocoagulation for refractory glaucoma postpenetrating keratoplasty. J Glaucoma 9(3):268–272
- ↑ 66.0 66.1 Subramaniam, K., Price, M. O., Feng, M. T., & Price, F. W. (2019). Micropulse Transscleral Cyclophotocoagulation in Keratoplasty Eyes. Cornea, 38(5), 542–545. doi:10.1097/ico.0000000000001897
- ↑ Lee JH, Shi Y, Amoozgar B, et al. Outcome of micropulse laser transscleral cyclophotocoagulation on pediatric versus adult glaucoma patients. J Glaucoma. 2017;26:936–939.
- ↑ Aquino MC, Bartan K, Tan AM, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43:40–46.