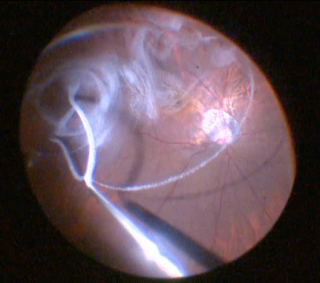
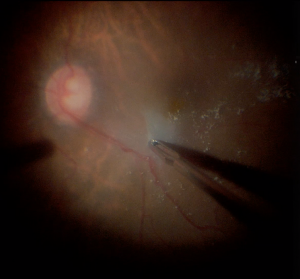
Chromovitrectomy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Vitreoretinal surgery is challenging, as microscopic pathology must be treated safely and delicately to maximize patient outcomes. Incremental technological innovations are constantly making vitreoretinal surgery safer and more efficient. Chromovitrectomy refers to the usage of vital dyes in vitreoretinal surgery to aid in the visualization of ocular tissues and anatomic planes. Specifically, the internal limiting membrane, vitreous humor, and epiretinal membranes are all semi-transparent ocular tissues that can be more easily visualized with vital dyes.
The internal limiting membrane (ILM) is the semitransparent basement membrane of the inner retina. The ILM is formed by the footplates of Müeller cells and is commonly removed in vitreoretinal interface diseases, such as macular hole and macular traction maculopathy.[1] Due to its thin (2 µm), transparent, and multilaminar nature, it is difficult to visualize and remove completely when performing ILM peels without the aid of vital dyes.[1]
The vitreous is a clear matrix of collagen, hyaluronate, and water. With a volume of approximately 5 mL, the vitreous fills the cavity between the lens and retina.[1] Vitrectomies are the mainstay of vitreoretinal surgeons, as removal allows access to performing a variety of procedures. Incomplete vitreous removal poses a risk, as complete removal better relieves retinal traction minimizing the risk of retinal tears and detachments. Staining allows easier visualization of remaining vitreous strands, ensuring a complete vitrectomy.
Epiretinal membrane (ERM) is a semi-translucent scar tissue found on the inner retina. ERMs form from an abnormal proliferation of glial cells and fibroblasts.[1] When affecting the macula, ERMs can cause traction, resulting in metamorphopsia and decreased vision. If visually significant, surgical ERM removal can be performed. Like the ILM and vitreous, ERMs can be hard to visualize and remove without the utilization of vital dyes.
Numerous studies have associated vital dye use with better postoperative outcomes.[2][3][4] To be an effective vital dye, a dye should provide sufficient staining, safety, easy visualization, and affordability. Many of the chromovitrectomy dyes currently used and under investigation are blue or green, since these colors provide the best contrast against the orange-red color of the retinal pigment epithelium (RPE). Vital dyes should be used judiciously as they are not without risks of toxicity. However, when used appropriately, dyes are a valuable tool for the vitreoretinal surgeon.
Indications
Chromovitrectomy is indicated in vitreoretinal procedures when it is important to accurately visualize the vitreous, ILM, or ERM. These procedures can include:
- Pars Plana Vitrectomy
- ILM Peel
- ERM Peel
- Macular Hole Repair
- Retinal Detachment Repair
- Anterior Vitrectomy
Contraindications
Vital dyes are contraindicated in patients with allergies to the selected dye or ingredients in the dye suspension. Staining formulations containing toxic ingredients and preservatives should also be avoided.
Surgical Technique
For vitreoretinal surgery, dyes are typically injected intravitreally prior to manipulation of the target tissues. There are multiple techniques for instilling dyes. The air-filled method consists of removing the intravitreal fluid via fluid-gas exchange before injecting dye toward the target area. It has the advantage of concentrating the dye in the posterior pole, achieving stronger staining and avoiding exposure of the peripheral retina and posterior capsule. The fluid-filled method consists of injecting dye into the intravitreal fluid with the convenience of not requiring fluid-gas exchange. However, this can result in a more diluted dye and exposure of larger areas of retina to potentially toxic dye.[5] This can be counteracted by injecting dye in a heavy suspension (such as in glucose solution) that sinks to the posterior pole. After a staining period, the dye is subsequently irrigated and washed out. Dyes can be reinjected later if staining is found to be inadequate.
Dyes
Triamcinolone Acetonide
Triamcinolone acetonide is a synthetic non-soluble corticosteroid that can be used to stain the cortical vitreous or ILM. In its usual preparations, triamcinolone acetonide is composed of white crystals in an aqueous suspension. These crystals are responsible for its staining ability, as they bind avidly to acellular tissues such as vitreous and also deposit on the ILM. The duration that triamcinolone acetonide remains in the eye depends on the specific formulation, but studies have found that crystals can remain in the vitreous cavity for up to 40 days after instillation.[6] The half life is around 18 days in nonvitrecomized eyes and 3 days in vitrectomized eyes.[7] Application of triamcinolone acetonide typically consists of injecting 0.1-0.3 mL with a concentration of 40 mg/mL. Triamcinolone acetonide has been shown to be more effective in highlighting vitreous than trypan blue, infracyanine green, and fluorescein.[8]
As a corticosteroid, triamcinolone acetonide has anti-inflammatory properties, and intravitreal injections are approved for the treatment of macular edema and uveitis. It has been speculated that usage of triamcinolone as a visualization tool may have the added benefit of decreasing postoperative inflammation and vitreoretinopathy. Triamcinolone acetonide has not been demonstrated to be toxic to the retina and remains a relatively safe option. There is a slightly increased risk in the development of cataracts and increased IOP with intravitreal triamcinolone use.
Commercial formulations of triamcinolone acetonide in the US are available as Triesence (Alcon) and Kenalog (Bristol-Meyers). Triesence is preservative-free, while Kenalog contains benzyl alcohol as a preservative. Animal studies have suggested benzyl alcohol can have a toxic effect on the neurosensory retina. Currently, only Triesence has FDA approval for intravitreal use. Usage of Kenalog for any indication intravitreally is considered off-label.
Trypan Blue
Trypan blue is a blue hydrophilic anionic azo dye that is generally used to stain ERMs, but also weakly stains the vitreous and ILM. Trypan blue is unable to cross cell membranes in living cells, and thus is only able to stain dead cells with compromised cell membranes. ERM contains a relatively high number of dead glial cells, and thus has a high affinity for trypan blue. Due to its hydrophilicity, trypan blue also has a weak affinity for the ILM and vitreous. Trypan blue is seldom used to only stain the ILM or vitreous, as other dyes (such as indocyanine green and corticosteroids) provide superior visualization.[9]
To apply trypan blue for ERM staining, a fluid-air exchange should first be performed to ensure the dye localizes to the macula. Afterwards, 0.15% concentration can be injected into the posterior pole. Alternatively, a 3:1 mixture of 0.15% trypan blue to 10% glucose can be used to create a dense solution that does not require fluid-air exchange before injection.
At typical concentrations, trypan blue is well tolerated by the retina.[10] At higher concentrations, histological analysis in a few animal and in vitro studies have demonstrated mild retinal toxicity.[11][12][13] Trypan blue is FDA approved for intraocular injection. It is available as MembraneBlue (Dutch Ophthalmic Research Center) at a concentration of 0.15%. This should not be confused with VisionBlue (Dutch Ophthalmic Research Center), which is used to stain the anterior capsule. VisionBlue has a lower concentration of 0.06%, which may be inadequate for vitreoretinal procedures.
Brilliant Blue
Brilliant blue is a blue anionic triarylmethane dye that has a strong affinity for the ILM. Brilliant blue is commonly used for ILM peeling, as it stains the ILM well without staining ERMs or vitreous. Adequate staining of the ILM can be achieved by applying brilliant blue at a concentration of 0.025% in an iso-osmolar solution towards the area of interest.
Most in vitro and in vivo studies have not yet shown retinal toxicity.[14] Atrophic changes of RPE have been reported when there is subretinal migration of the dye.[15] Many studies seem to suggest a better safety profile for brilliant blue compared to indocyanine green.[16] Thus, brilliant blue may present a better option for ILM staining.
Brilliant blue is currently approved for use in Europe as Brilliant Peel (Fluoron), and has received FDA approval and available in the US as DORC International’s TissueBlue as pre-mixed pre-filled syringe with 0.025% solution.
Bromophenol Blue
Bromophenol blue is a blue triarylmethane dye that has high affinity for the ILM and ERMs. Bromophenol blue can be injected at a concentration of 0.13-0.2% and does not require fluid-gas exchange. In vitro studies have demonstrated low toxicity to the retina with significantly less toxic effects than indocyanine green.[17] Studies in human subjects injected with intraocular bromophenol blue have also not shown any functional visual deficits as measured through perimetry and ERG.[18]
Given the favorable safety profile, bromophenol blue is another dye that may be preferable to indocyanine green for ILM staining. It is currently approved for use in Europe and commercially available in a combined formulation with brilliant blue known as Brilliant Peel Dual Dye (Fluoron). It is not FDA-approved.
Patent Blue
Patent blue is another blue anionic triarylmethane dye that is being investigated as a potential stain for ERMs. Like trypan blue, patent blue has moderate affinity for ERMs and a low affinity for the ILM.[19] Patent blue can be injected towards the area of interest in an iso-osmolar solution at 0.25% concentration or diluted in 0.25% glucose.
Most studies suggest that patent blue has a similar safety profile to trypan blue when used in the vitreous space. Animal studies have demonstrated low toxicity of patent blue when injected subretinally, and in vitro models have not shown any irreversible effect on retinal function.[20][21] Surgical case reports have not reported any postoperative visual field defects or grossly visible RPE changes. Patent blue is currently approved in Europe for capsular staining during cataract surgery under the brand name of Blueron® (Fluoron). Its use in vitreoretinal surgery is off-label, and it does not yet have FDA approval.
Indocyanine Green and Infracyanine Green
Indocyanine green (ICG) is a green anionic cyanine dye that is useful for ILM staining. It has high affinity for components of the extracellular matrix present in the ILM such as collagen type IV and laminin. In porcine studies, ICG causes increased stiffness of the ILM when it is exposed to light, possibly due to alterations in collagen structure.[22] This stiffening may facilitate easier removal in one piece with less damage to underlying tissues. Various studies have shown improved ease of ILM removal and less trauma when ICG is applied before ILM peeling.[23] ICG requires initial dilution in distilled water. If diluted initially in saline, the dye can precipitate. The diluted solution should then be subsequently diluted in balanced saline solution to create a less hypo-osmolar solution.
There are some concerns over ICG’s toxicity when used intraocularly. A meta-analysis of 837 eyes showed similar anatomical outcomes when using ICG versus no dye in ILM peels, but worse functional outcomes.[24] The authors implicated dye-induced toxicity as a possible culprit. Numerous animal studies have shown that ICG is toxic to chorioretinal cells when exposed to moderate to high concentrations. ICG has also been proposed to cause toxicity when applied and exposed to light through a photochemical mechanism. Studies performed in rabbits resulted in RPE changes and functional retinal damage when subretinal ICG injections were exposed to light.[25][26] The FDA has approved ICG for intravenous use in angiography, but not for intraocular use. Thus, its use in vitreoretinal surgery is currently off-label.
Infracyanine green (IFCG) is another green anionic cyanine dye that is very chemically and pharmacologically similar to indocyanine green. Like ICG, it primarily stains the ILM.[27] Its main difference is that it does not contain sodium iodide unlike ICG (ICG contains 4-5% iodine in part as a byproduct of its synthesis and in part due to the presence of an iodine moiety on the molecule). Thus, it has the advantage of not requiring initial dilution in distilled water to avoid precipitation. It also avoids exposure of the retina to toxic iodide. More study is still needed to determine its toxicity.
Sodium Fluorescein
Sodium fluorescein is a yellow fluorescent xanthene dye that has many ophthalmic applications including acting as a stain for the vitreous. Sodium fluorescein is highly hydrophilic and is well absorbed by the vitreous due to its high water content. Its benefits include its low toxicity and low cost. However, fluorescein is generally inferior to triamcinolone acetonide in its ability to highlight vitreous tissue and thus, is less commonly used.[8]
Fluorescein is currently FDA-approved for topical and intravenous use only and is available in many commercial formulations. Intraocular use is off-label.
Complications and Toxicity
As with the injection of any intraocular substance, toxicity is a major concern. Toxicity may result from the pharmacologic effects of the dyes themselves or they may result from the vehicles and suspensions that dyes are delivered in. These vehicles can alter the osmolarity, pH, and ionic composition in the vitreous space to non-physiologic levels that can induce toxic effects on the retina. Both hyper-osmotic and hypo-osmotic solutions can induce toxicity when injected into the vitreous space. Hyper-osmotic solutions have been shown to alter the cellular architecture of the retina and induce shrinkage of retinal layers.[28] Animal studies in which eyes are injected with hyper-osmotic solution leads to increased rates of retinal detachments and edema of the RPE.[29] Hypo-osmotic solutions can be toxic to the retina as well. The mechanism is not completely understood, but may be related to an influx of intracellular calcium and upregulation of enzymatic activity within RPE cells. Some of the toxicity attributed to ICG may be due to cases of injecting with a hypo-osmolar solution. Care should be taken to ensure that only iso-osmolar and pH-balanced dye solutions are injected intraocularly.
In addition to altering osmolarity and pH, vehicles for dyes can contain preservatives and additives that have their own deleterious effects. A frequently used commercial preparation of methylprednisolone acetate, Depo-Medrol (Pfizer), contains the preservative myristyl-𝛾-picolinium chloride. This preservative has been shown to be highly toxic to the retina. Animal studies in which eyes have been injected with myristyl-𝛾-picolinium chloride have shown a permanently decreased ERG response even after many months.[30] Depo-Medrol only has FDA approval for periocular use as an anti-inflammatory agent, but does not have approval for intraocular use. Multiple case reports exist in which Depo-Medrol was unintentionally injected intraocularly or intentionally injected to stain vitreous by those unaware of its effects.[31][32] Retinal necrosis and various toxic sequelae were seen as a result in these instances. Given the toxic nature of myristyl-𝛾-picolinium chloride, Depo-Medrol and other formulations containing similar preservatives should never be used for intraocular staining.
Benzyl alcohol is another common preservative that is found in certain formulations of triamcinolone acetonide, including Kenalog. The data on the toxicity of benzyl alcohol at concentrations found in commercial formulations is still unclear. In animal studies comparing the effects of injecting triamcinolone acetonide formulations with benzyl alcohol (Kenalog) vs those without, severe retinal damage and histological disorganization of retinal layers were seen in the rabbit eyes injected with Kenalog.[33] However, other animal studies have shown no observable toxic effects.[34][35] A small prospective study on human eyes did not find measurable signs of visual deterioration when Kenalog was injected intravitreally.[36] Until further data is obtained, it may be safer to use preservative-free formulations that are FDA-approved for intraocular use such as Triessence instead of those that contain preservative.
Summary Table
| Dye | Concentration | Tissues Stained | Cost | Toxicity | Availability in the US |
|---|---|---|---|---|---|
| Triamcinolone Acetonide | 40 mg/mL (4%) | Vitreous and ILM | $ |
|
|
| Trypan Blue | 1.5 mg/mL (0.15%) | High affinity for ERM. Low affinity for ILM. | $ |
|
|
| Brilliant Blue | 0.25 mg/mL (0.025%) | ILM | $$ |
|
|
| Bromophenol Blue | 1.3 mg/mL (0.13%) | ILM and ERM. | $$ |
|
|
| Patent Blue | 2.4 mg/mL (0.24%) | Moderate affinity for ERM. Low affinity for ILM. | $$ |
|
|
| Indocyanine Green | Dilute to <0.5 mg/mL | ILM | $$$ |
|
|
| Infracyanine Green | Dilute to <0.5 mg/mL | ILM | $$$$ |
|
|
| Sodium Fluorescein | Variable | Vitreous | $ |
|
|
References
- ↑ 1.0 1.1 1.2 1.3 Schachat, Andrew P. Ryan's Retina. Elsevier, 2018.
- ↑ Mester V, Kuhn F. Internal limiting membrane removal in the management of full thickness macular holes. Am J Ophthalmol. 2000;129(6):769--77
- ↑ Lochhead J, Jones E, Chui D, et al. Outcome of ICG-assisted ILM peel in macular hole surgery. Eye. 2004;18(7):804--8
- ↑ Haritoglou C, Eibl K, Schaumberger M, et al. Functional outcome after trypan blue assisted vitrectomy for macular pucker: a prospective, randomized, comparative trial. Am J Ophthalmol. 2004;138(1):1--5
- ↑ Rodrigues, Eduardo, et al. “The Use of Vital Dyes in Ocular Surgery.” Survey of Ophthalmology, vol. 54, no. 5, Sept. 2009, pp. 576–617.
- ↑ Fukukita M, Sasoh M, Matsubara H, et al. Triamcinolone acetonide remaining on the fovea after successful macular hole closure. Retina. 2007;27(1):122--3
- ↑ Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110(4):681-686.
- ↑ 8.0 8.1 Guo S, Tutela AC, Wagner R, Caputo AR. A comparison of the effectiveness of four biostains in enhancing visualization of the vitreous. J Pediatr Ophthalmol Strabismus. 2006;43(5):281--4
- ↑ Lee KL, Dean S, Guest S. A comparison of outcomes after indocyanine green and trypan blue assisted internal limiting membrane peeling during macular hole surgery. Br J Ophthalmol. 2005;89(4):420--4
- ↑ Stalmans P, Van Aken EH, Melles G, et al. Trypan blue not toxic for retinal pigment epithelium in vitro. Am J Ophthalmol. 2003;135(2):234--6
- ↑ Rezai KA, Farrokh-Siar L, Gasyna EM, Ernest JT. Trypan blue induces apoptosis in human retinal pigment epithelial cells. Am J Ophthalmol. 2004;138(3):492--5
- ↑ Kwok AK, Yeung CK, Lai TY, et al. Effects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cells. Br J Ophthalmol. 2004;88(12):1590--4
- ↑ Luke C, Luke M, Dietlein TS, et al. Retinal tolerance to dyes. Br J Ophthalmol. 2005;89:1188--9
- ↑ Enaida H, Hisatomi T, Goto Y, et al. Preclinical investigation of internal limiting membrane staining and peeling using intravitreal brilliant blue G. Retina. 2006;26(6): 623--30
- ↑ Malerbi F, Maia M, Farah ME, et al. Subretinal Brilliant blue migration during epiretinal membrane peeling. BJO 2009. Forthcoming.
- ↑ Enaida H, Hisatomi T, Hata Y, et al. Brilliant blue G selectively stains the internal limiting membrane/brilliant blue G-assisted membrane peeling. Retina. 2006;26(6): 631--6
- ↑ Balaiya, Sankarathi, et al. “Comparative In Vitro Safety Analysis Of Dyes For Chromovitrectomy.” Retina, vol. 31, no. 6, 2011, pp. 1128–1136., doi:10.1097/iae.0b013e3181fe543a.
- ↑ Haritoglou, C., et al. “Vitreoretinal Surgery Using Bromphenol Blue as a Vital Stain: Evaluation of Staining Characteristics in Humans.” British Journal of Ophthalmology, vol. 91, no. 9, 2007, pp. 1125–1128., doi:10.1136/bjo.2007.115113.
- ↑ Mennel S, Meyer CH, Tietjen A, et al. Patent blue: a novel vital dye in vitreoretinal surgery. Ophthalmologica. 2006; 220(3):190--3
- ↑ Maia M, Penha F, Rodrigues EB, et al. Effects of subretinal injection of patent blue and trypan blue in rabbits. Curr Eye Res. 2007;32(4):309--17
- ↑ Lüke, C., et al. “Effects of Patent Blue on Human Retinal Function.” Graefe's Archive for Clinical and Experimental Ophthalmology, vol. 244, no. 9, 2006, pp. 1188–1190., doi:10.1007/s00417-005-0239-5.
- ↑ Wollensak G, Spoerl E, Wirbelauer C, Pham DT. Influenc of indocyanine green staining on the biomechanical strength of porcine internal limiting membrane. Ophthalmologica. 2004;218(4):278--82
- ↑ Da Mata AP, Burk SE, Foster RE, et al. Long-term follow-up of indocyanine greenassisted peeling of the retinal internal limiting membrane during vitrectomy surgery for idiopathic macular hole repair. Ophthalmology. 2004;111(12): 2246--53
- ↑ Rodrigues EB, Meyer CH. Meta-analysis of chromovitrectomy with indocyanine green in macular hole surgery. Ophthalmologica. 2008;222(2):123--9
- ↑ Maia M, Margalit E, Lakhanpal R, et al. Effects of intravitreal indocyanine green injection in rabbits. Retina. 2004;24(1):69--79
- ↑ Maia M, Kellner L, de Juan E Jr, et al. Effects of indocyanine green injection on the retinal surface and into the subretinal space in rabbits. Retina. 2004;24(1):80--91
- ↑ Ullern M, Roman S, Dhalluin JF, et al. Contribution of intravitreal infracyanine green to macular hole and epimacular membrane surgery: preliminary study. J Fr Ophtalmol. 2002;25(9):915--20
- ↑ Hida T, Chandler D, Arena JE, et al. Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. Am J Ophthalmol 1986;101:190-195
- ↑ Negi A, Marmor MF: Experimental serous retinal detachment and focal pigment epithelial damage. Arch Ophthalmol 1984; 102: 445–449.
- ↑ Loewenstein A, Zemel E, Lazar M, Perlman I: The effects of Depo-Medrol preservative on the rabbit visual system. Invest Ophthalmol Vis Sci 1991; 32: 3053–3060.
- ↑ Brill, Daniel A., et al. “Methylprednisolone Acetate (Depo-Medrol) Injection during Cataract Surgery Causing Retinal Necrosis.” Ophthalmology, vol. 126, no. 9, 2019, pp. 1332–1333., doi:10.1016/j.ophtha.2019.03.034.
- ↑ Yonekawa, Yoshihiro, et al. “Retinal Necrosis Secondary To Inadvertent Intravitreal Methylprednisolone Acetate (Depo-Medrol) Injection During Pars Plana Vitrectomy.” Retinal Cases & Brief Reports, vol. 3, no. 4, 2009, pp. 336–339., doi:10.1097/icb.0b013e318173778d.
- ↑ Lang Y, Zemel E, Miller B, Perlman I: Retinal toxicity of intravitreal Kenalog in albino rabbits. Retina 2007; 27: 778–788.
- ↑ Hida T, Chandler D, Arena JE, et al. Experimental and clinical observations of the intraocular toxicity of commercial corticosteroid preparations. Am J Ophthalmol 1986;101:190-195
- ↑ Dierks D, Lei B, Zhang K, et al. Electroretinographic effects of an intravitreal injection of triamcinolone in rabbit retina. Arch Ophthalmol 2005
- ↑ Lang Y, Leibu R, Shoham N, Miller B, Perlman I: Evaluation of intravitreal Kenalog toxicity in humans. Ophthalmology 2007; 114: 724–731.