Choroidal Rupture
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
A choroidal rupture is a break in the choriocapillaris, Bruch's membrane, and the retinal pigment epithelium (RPE).
Disease
A choroidal rupture occurs as a result of traumatic mechanical event directly at the site of contusion or, more commonly, away from the impact. The force buckles the globe in at the area of impact and causes stress folding of the globe wall at a peripheral site causing the choroid, RPE and Bruch membrane complex to stretch and break.
Etiology
A choroidal rupture usually is a result of a traumatic injury; it is noted three times more frequently with closed globe injuries than open globe. It is noted with both blunt and penetrating globe injuries[1].
Risk Factors
Any type of ocular trauma is a risk factor for the development of a choroidal rupture. Sports injuries due to martial arts, basketball, soccer, tennis, basketball, golf, hockey puck, paint balls have all been reported in the literature. Car accidents with air bag injuries have led to choroidal ruptures. Although not associated with systemic disease, eyes of patients with pseudoxanthoma elasticum, Ehlers-Danlos and angioid streaks have brittle Bruch membrane and are prone to rupture with minimal trauma. In a large retrospective study of 101 patients with choroidal rupture, the mean age was 36 years, and 76% were men[2].
General Pathology
Histopathologically, a choroidal rupture demonstrates a disruption in the choriocapillaris, the RPE, and Bruch's membrane. The overlying neurosensory retina is intact.
Pathophysiology
During a closed globe injury, the eyeball is first mechanically compressed and then rapidly hyperextended. The sclera's tensile strength resists this compression. The retina is elastic and stretches during such an injury. However, Bruch's membrane breaks because it does not have sufficient tensile strength or elasticity. The choriocapillaris is injured and bleeds into the subRPE and/or subretinal space. Such hemorrhage may hide the choroidal rupture initially. Over days, the blood clears and a whitish/yellowish, curvilinear, crescent-shaped subretinal streak is visible, usually concentric to the optic disc. Over time, choroidal neovascularization (CNV) can develop. In most, the CNV involutes over time. In about 30% of cases, the CNV may recur, with a serous or hemorrhagic pigment epithelial detachment, anytime following formation of the choroidal rupture. If the rupture or CNV does not involve the foveal center, vision may not be affected.
Primary Prevention
In order to prevent the development of a choroidal rupture, avoidance of a closed globe injury is necessary. Avoiding high risk activities and wearing polycarbonate eye protection may prevent the development of a choroidal rupture.
Diagnosis
The diagnosis of a choroidal rupture is made based on the history of a closed globe injury in an eye with a crescent shaped yellowish/whitish subretinal streak that is usually concentric with the optic disc.
History
A history of recent or remote blunt trauma resulting in a closed globe injury to the eye is a necessary component to the history in order to make the diagnosis of a choroidal rupture.
Physical Examination
A complete ocular examination is needed and would include a dilated examination of the fundus to detect the choroidal rupture(s).
Signs
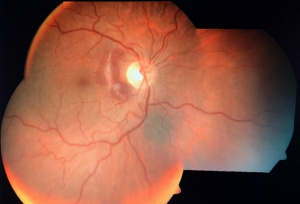
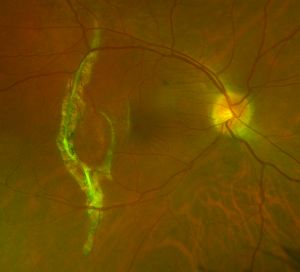
A choroidal rupture may be evident in the posterior pole as a white or yellow crescent-shaped streak that is subretinal in location. It is usually concentric to the optic nerve (Figure 1). There may be one or more choroidal ruptures present. Initially after a closed globe injury, the rupture may not be evident to the physician because it may be obscured by associated hemorrhage. As the blood reabsorbs, the choroidal rupture may be observed. In rare cases, the choroidal rupture may be oriented radially. Choroidal neovascularization (CNV) may develop from the choroidal rupture. The choroidal rupture may be partial thickness or full thickness. According to the mechanism of development either direct or indirect choroidal ruptures can be seen. Direct choroidal rupture is seen at the peripheral fundus (mostly temporal) at the site of impact parallel to the ora serrata. Indirect choroidal ruptures are seen at the posterior pole due to a countercoup effect of the trauma. Indirect choroidal ruptures are more common. Associated trauma to globe, orbital bones, and retinal dialyses should be ruled out.
Symptoms
Symptoms of a choroidal rupture are dependent on the location of the rupture within the eye. An individual with a choroidal rupture may indeed be asymptomatic if the rupture and any associated hemorrhage does not involve the fovea or parafoveal retina. If the rupture and/or hemorrhage involves the fovea or adjacent retina, decreased vision may be the first symptom noted. A large study found that the initial visual acuity was 20/225, improving to 20/135 at last follow-up[2].
Clinical Diagnosis
The clinical diagnosis of a choroidal rupture can be made during ophthalmoscopic examination of the fundus. The location of choroidal rupture has been reported as being foveal, extrafoveal, and extramacular in 30%, 45%, and 25% of cases, respectively[2].
Diagnostic procedures
- Fluorescein angiographic findings typically shows hypofluorescence in early frames due to a break in choriocapillaris and choroidal vessels at the rupture site with staining at late frames due to dye leakage from adjacent choriocapillaris, If CNV is present, hyperfluoresence that progressively increases in size and intensity will be seen with time.
- Autofluorescence of the rupture site will show hypofluorescence of the wound site where RPE is missing with hyperfluoresence at the edge of choroidal rupture.
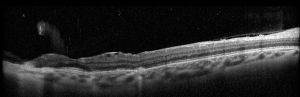
- OCT shows loss of continuity of RPE at the site of rupture with thinning of underlying inner choroid.
Laboratory test
No laboratory evaluation is necessary since eyes with choroidal rupture have a history of recent or remote blunt trauma to that eye.
Differential diagnosis
Lacquer cracks in an eye with high myopia are in the differential diagnosis of choroidal rupture. Lacquer cracks are often bilateral and the eyes have other signs of high myopia including a scleral crescent adjacent to the tilted disc and a posterior staphyloma. Angioid streaks are often in the differential diagnosis and are bilateral subretinal streaks that emanate from the optic disc and may be associated with CNV.
Management
Observation is recommended. An Amsler grid may be given to the patient and any changes in the grid should be reported to the patient's eye care provider since a change may indicate the development of CNV. Patients should be counseled that CNVs develop in 7.9% of choroidal rupture cases (8 of 101 eyes)[2]. The majority of CNVs (88%) are associated with foveal ruptures[2].
General treatment
Observation is recommended with Amsler grid monitoring. However, in the presence of CNV, anti-vascular endothelial growth factor (VEGF) injections can be used. However, Compared to exudative age-related macular degeneration, it is likely that fewer injections would be needed to control a choroidal rupture-related CNV[3]. The mean interval between the ocular trauma and the diagnosis of CNV was 5.7 months in one study (SD 4.75; range 2-12) with an average of 4.2 injections (SD 3.2; range 1-8) performed per eye[3]. However, a larger study noted a patient who required 40 eye injections for recurrent exudation[2]. Expectant management may be a viable treatment option even for CNV based on location and vision prognosis[4]. Photodynamic therapy (PDT) has been reported as an adjunct therapy to anti-VEGF in the setting of CNV for choroidal ruptures[2].
Medical therapy
There is no medical therapy available to cause resolution of a choroidal rupture.
Medical follow up
Follow-up is indicated one to two times per year or as dictated by symptoms and related findings (e.g., CNV).
Surgery
There is no surgical procedure available that would result in resolution of a choroidal rupture. Isolated case reports of successful management of recent onset thick subretinal hemorrhage at fovea with intravitreal gas (SF6 or C3F8), tissue plasminogen activator (rtPA) exist in literature
Complications
Subretinal and sub-RPE hemorrhage can result at the time the choroidal rupture develops.
Choroidal neovascularization, usually type 2, can develop from the choroidal rupture where choroidal neovessels proliferate and grow in the subretinal space, leading to hemorrhage and fibrosis and decreased vision if untreated. Most CNV undergo spontaneous resolution, but many will need anti-VEGF injection.
Prognosis
A choroidal rupture by itself does not change over time. Visual acuity at presentation depends on the location of the rupture and presence of retinal edema and hemorrhage in the macula. The vision may improve as the subretinal edema and hemorrhage improves. However, there is a risk of CNV formation due to the rupture in Bruch membrane and growth of CNV in the subfoveal area could lead to decreased central vision in an eye where the fovea was spared by the choroidal rupture. Subfoveal ruptures and associated traumatic optic neuropathy denote a poor visual prognosis.
Additional Resources
References
- ↑ Lupidi M, Muzi A, Castellucci G, Kalra G, Piccolino FC, Chhablani J, Cagini C. The choroidal rupture: current concepts and insights. Surv Ophthalmol. 2021 Sep-Oct;66(5):761-770. doi: 10.1016/j.survophthal.2021.01.014. Epub 2021 Feb 2. PMID: 33545177.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Russell JF, Albini TA, Berrocal AM, Dubovy SR, Rosenfeld PJ, Smiddy WE, Sridhar J, Townsend JH, Flynn HW Jr. Anti-Vascular Endothelial Growth Factor Therapy for Choroidal Rupture-Associated Choroidal Neovascularization. Ophthalmol Retina. 2020 Feb;4(2):226-228. doi: 10.1016/j.oret.2019.09.008. Epub 2019 Sep 21. PMID: 32033715.
- ↑ Jump up to: 3.0 3.1 Barth T, Zeman F, Helbig H, Gamulescu MA. Intravitreal anti-VEGF treatment for choroidal neovascularization secondary to traumatic choroidal rupture. BMC Ophthalmol. 2019 Nov 26;19(1):239. doi: 10.1186/s12886-019-1242-7. PMID: 31771544; PMCID: PMC6878647.
- ↑ Adhi M, Reinoso M. Spontaneous regression and quiescence of choroidal neovascularization secondary to traumatic choroidal rupture depicted on OCT angiography. Eur J Ophthalmol. 2021 Nov 17:11206721211059030. doi: 10.1177/11206721211059030. Epub ahead of print. PMID: 34787002.