Choroidal Neovascularization Secondary to Chorioretinitis/Posterior Uveitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease
International Classification of Disease (ICD) Diagnosis Code
Choroidal neovascularization secondary to chorioretinitis (H35.059, H30.90).[1]
Disease Entity
Inflammatory choroidal neovascularization (I-CNV) is a potentially vision-threatening complication of chorioretinal inflammation in which blood vessels originating from the choroid break through Bruch’s membrane as a neovascular complex [2] beneath the retinal pigment epithelium (Type 1 CNV) or above the retinal pigment epithelium (Type 2 CNV). These I-CNV cause vision loss by exuding serous fluid, blood, or lipid under or into the retina. While a history of posterior uveitis may aid in establishing this diagnosis, inflammatory CNV may be the first presenting sign of posterior uveitis. Inflammatory CNV can be subdivided by their anatomic location (macular, peripapillary, or peripheral) and categorized as secondary to specific types of chorio-retinal inflammation.
Pathophysiology
There are two mechanisms by which posterior uveitis may promote the development of I-CNV [3]:
(1) Inflammatory mediated damage of the Bruch’s membrane-retinal pigment epithelium complex disrupts the outer blood-retinal barrier and permits neovascular upgrowth from the choroid.
(2) Inflammation of the retina and choroid compromises perfusion, generating a gradient of retinal-choroidal hypoxia that promotes neovascularization under or into the retina.
The underlying pathophysiology of I-CNV is likely similar to the pathophysiology of CNV seen in other conditions associated with CNV such as age-related macular degeneration [4] or pathologic myopia. [5] I-CNV may thus be considered not as a distinct form of CNV, but rather associated with a set of circumstances which permit their development. Distinguishing I-CNV from other forms of CNV may be useful because of the implications for management of this condition as well as the associated uveitis.
There have been many growth factors identified in the development of CNVMs including Vascular Endothelial Growth Factor (VEGF) and basic fibroblast growth factor (bFGF) [6]. VEGF expression promotes development of novel blood vessel growth and strongly correlates with the development of CNVM.[7] [8]
Risk Factors [3] [9]
The prevalence of I-CNV among the various causes of posterior uveitis varies widely as seen in Table 1. I-CNV can be seen in Toxoplasma chorioretinitis but are rare in ocular tuberculosis. Among the non-infectious posterior uveitides, I-CNV is a hallmark of punctate inner choroidopathy but less common in Vogt-Koyanagi-Harada syndrome, which can be associated with panuveitis. Hence, the degree of intraocular inflammation may not necessarily correlate with the likelihood of I-CNV development.
| Non-Infectious Posterior Uveitis | Prevalence of CNV (%) | Infectious Posterior Uveitis | Prevalence of CNV (%) |
|---|---|---|---|
| Punctate Inner Choroidopathy | 75% | Toxoplasmosis | 0.3-19% |
| Multifocal Choroiditis | 33-50% | Intraocular Tuberculosis | Uncommon |
| Serpiginous Choroiditis | 10-25% | ||
| Vogt-Koyanagi-Harada (VKH) Syndrome | 9-15% |
Diagnosis
Differential diagnosis
I-CNV are not so much a distinct form of CNV, but rather as a unique set of circumstances in which CNV may develop. Distinguishing I-CNV from other forms of CNV may be useful because of the implications for management of the uveitis as well as the CNV. While CNV secondary to age related macular degeneration (AMD),[10] chronic central serous retinopathy (CSR),[11] pathologic myopia, trauma, or angioid streaks may be more common than I-CNV, identifying the associated uveitis can aid in short and potentially long term treatment of the underlying inflammation.
Symptoms
- Painless vision loss or scotomas
- Metamorphopsia
- Photopsias (especially with active uveitis)
Signs
Careful funduscopic examination can reveal CNV around the optic nerve (peripapillary), in the macula, as well as in the periphery. These lesions are typically subretinal in location and yellow-white in color. They may have associated pigment, fluid, lipid, or blood. Additional clues in inflammatory CNV can include vitreous cell or haze, optic disc edema, hypopigmented or pigmented spots in macula or periphery and macular or peripheral serous retinal detachments. There may also be signs of prior CNV activity often seen as fibrovascular scars with or without exudation.
Imaging
I-CNV can be identified by fundus imaging modalities including optical coherence tomography (OCT) and fluorescein angiography (FA). CNV secondary to posterior uveitis, which are less common than other causes of CNV, are often diagnosed by utilizing these modalities with indocyanine green angiography (ICGA), and fundus autofluorescence (FAF). As mentioned previously, inflammatory CNV can be subdivided by their anatomic location with the macular being the most common location. In a retrospective analysis of 20 eyes of 17 patients with I-CNV, 60% developed sub-foveal I-CNV, 35% developed juxtafoveal I-CNV, and only 5% developed peripapillary I-CNV.[12] It is important to consider that peripheral I-CNV may be under-reported as these are less likely to cause vision threatening symptoms.
Frequently observed features on optical coherence tomography (OCT) include disruption of the ellipsoid zone, choroidal hypertransmission, absence or atrophy of the retinal pigment epithelium (RPEA), the presence of hyperreflective foci (HRF) within the retina, engorgement of the choroidal vessels, focal excavation of the choroid, and irregular vascular loops (as seen on OCT angiography).[13]
Macular I-CNV
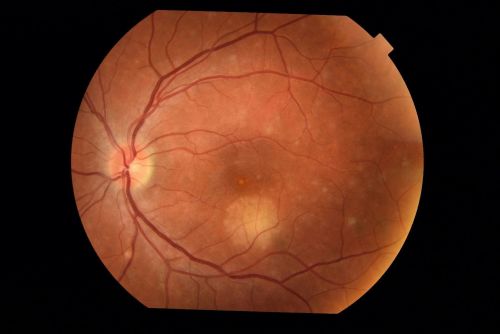
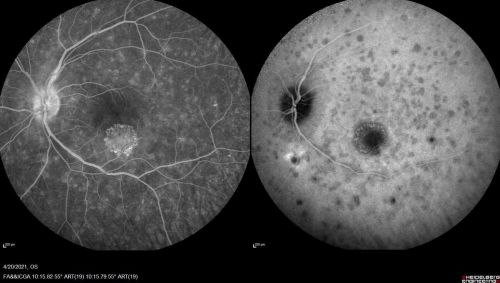
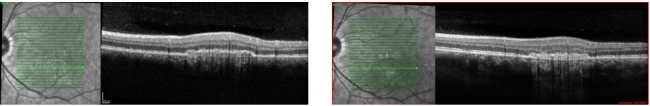
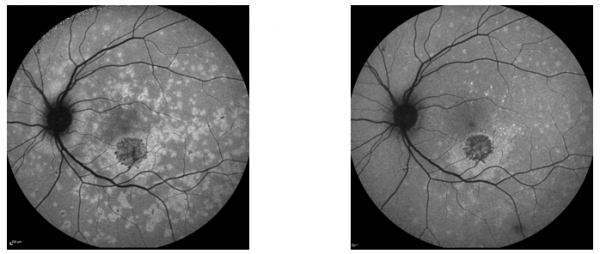
Peripapillary I-CNV
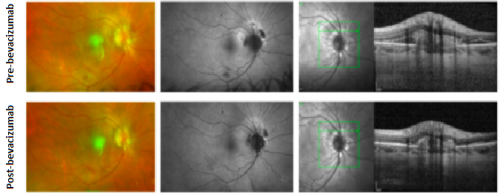
Peripheral I-CNV
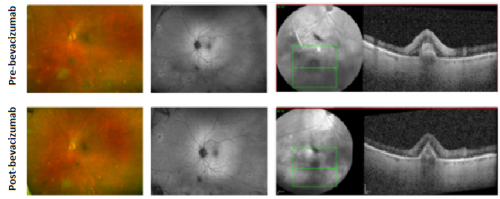
Management
Prompt treatment of I-CNV can prevent irreversible vision loss. Following initial treatment, diligent surveillance is necessary to identify and treat recurrences. Management of I-CNV typically requires a two-pronged approach:
- Diagnosis (if not previously identified) and treatment of the underlying uveitis
- Intravitreal anti-VEGF injections for the I-CNV
Management of underlying uveitis
A full discussion of the diagnosis and treatment of the uveitides that cause I-CNV is beyond the scope of this article. In brief, the diagnostic approach for a patient with new uveitis should align with several key priorities [14]:
- Rule out infectious conditions that might worsen without proper anti-infective treatment (e.g. Toxoplasma chorioretinitis, Tuberculous uveitis)
- Identify systemic conditions (e.g. Sarcoidosis or VKH), the care of which may require collaboration with other specialists
- Utilize imaging to help classify one of the common uveitis phenotypes
Once the appropriate conditions have been ruled out, management of the underlying uveitis typically involves systemic steroids (after any necessary anti-infective therapy has been initiated) in the short term. Some of the conditions associated with I-CNV may be recurrent, chronic, or progressive (Multifocal Choroiditis, Vogt-Koyanagi-Harada Syndrome, Serpiginous Choroiopathy). In patients with I-CNV associated with these conditions, steroid-sparing immunosuppressive therapy might be considered to reduce the likelihood of additional ocular structural damage and vision loss.
Periocular or regional corticosteroid injections may be used in addition to or in place of systemic treatment of uveitis associated with I-CNV. In one study, a combination of anti-inflammatory therapy (oral or intravenous) and intravitreal anti-VEGF treatment had an improved outcome for 80% of patients and stable outcome for 15%.[15] The decision of systemic and/or regional steroid therapy should be tailored based on whether one or both eyes are affected, age of the patient, and risk of cataract development or ocular hypertension, and systemic health considerations including oral corticosteroid intolerance and systemic hyperglycemia. [16]
Management of the I-CNVM
Intravitreal injections of anti-vascular endothelial growth factor (VEGF) medications such as bevacizumab, ranibizumab, or aflibercept are highly effective against I-CNVMs.[17] [15] One study compared two different treatment regimens to determine the best anti-VEGF treatment: a group that was given monthly injections for three months as a loading phase then re-treated as needed (Loading group) and a group that was treated as needed from the start (PRN group). The study found that the loading phase did not result in superior outcomes.[18] Photodynamic therapy (PDT) and thermal laser photocoagulation have largely been supplanted by anti-VEGF injections,[19] as has been the case with other types of CNV.
Monitoring and Prognosis
Treatment of I-CNV with control of the underlying uveitis and intravitreal anti-VEGF agents can help to preserve and improve vision. One summary of the literature on long-term outcomes after anti-VEGF treatment of I-CNV included studies with follow up of time periods ranging from six months to 5 years with a variety of underlying posterior uveitic etiologies. Among these studies, improvement in logMAR visual acuity of approximately 0.3 units was common after anti-VEGF therapy (between 1-5 injections) [20] In one retrospective case series of individuals with PIC who were treated with anti-VEGF injections without systemic therapy, there was a 50% recurrence rate. [21]
Multimodal imaging can enhance the sensitivity of early detection as well as monitoring of I-CNV. OCT volume scans of the macula and optic nerve may be very useful for identifying vision threatening macular or peripapillary I-CNV. Fundus autofluorescence can enable detection of peripheral I-CNV. Fluorescein and ICG angiography as well as OCT angiography may be helpful for monitoring both the underlying uveitis as well as the I-CNV. It is important to remember that while a gradual extension of the anti-VEGF injection interval may work well for most types of CNV, patients with I-CNV should have their uveitis monitored regularly even when CNV activity can be halted as the uveitis will follow its own time course. Subsequent uveitis flares may/may not correspond with CNV reactivation, so each aspect needs careful surveillance.
References
- ↑ https://www.icd10data.com/ICD10CM/Codes/H00-H59/H30-H36/H35-/H35.059
- ↑ [Turbert, D. (2020, March 18). What are Choroidal Neovascular membranes? Reviewed by Ninel Z Gregori, MD. American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/choroidal-neovascular-membranes.].
- ↑ Jump up to: 3.0 3.1 3.2 [Agarwal A. et al. “An update on inflammatory choroidal neovascularization: epidemiology, multimodal imaging, and management”. J Ophthalmic Inflamm Infect. Vol: 8(13). doi: 10.1186/s12348-018-0155-6]
- ↑ [Kauppinen A. et al. “Inflammation and its role in age-related macular degeneration”. Cell Mol Life Sci. 2016; 73: 1765–1786. Feb 6, 2016.]
- ↑ [Cheung C. et al. “Myopic Choroidal Neovascularization: Review, Guidance, and Consensus Statement on Management”. Ophthalmology. 2017 Nov;124(11):1690-1711. doi: 10.1016/j.ophtha.2017.04.028.]
- ↑ [Soubrane G. et al. “Basic fibroblast growth factor experimentally induced choroidal angiogenesis in the minipig”. Curr Eye Res. 1994 Mar;13(3):183-95. doi: 10.3109/02713689408995776.]
- ↑ [Spillsbury K. et al. “Overexpression of Vascular Endothelial Growth Factor (VEGF) in the Retinal Pigment Epithelium Leads to the Development of Choroidal Neovascularization”. Am J Pathol. 2000 Jul; 157(1): 135–144. doi: 10.1016/S0002-9440(10)64525-7]
- ↑ [Frank R. et al. “Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes”. Am J Ophthalmol. 1996 Sep;122(3):393-403. doi: 10.1016/s0002-9394(14)72066-5.]
- ↑ Patel A. et al. “Risk of Retinal Neovascularization In Cases of Uveitis”. Ophthalmology. Vol: 123(3). Pg: 646–654. doi: 10.1016/j.ophtha.2015.10.056]
- ↑ [Chang L. et al. (Jul 2008). “Bevacizumab treatment for subfoveal choroidal neovascularization from causes other than age-related macular degeneration”. JAMA Ophthalmology, 126(7): pg 941-945. doi: 10.1001/archopht.126.7.941]
- ↑ [Filho M et al. (Aug 2015). Association of Choroidal Neovascularization and Central Serous Chorioretinopathy With Optical Coherence Tomography Angiography. JAMA Ophthalmology. 133(8):899-906, doi: 10.1001/jamaophthalmol.2015.1320.]
- ↑ [Jabs and Busingye. “Approach to the diagnosis of the uveitides”. Am J Ophthalmol. 2013 Aug;156(2):228-36. doi: 10.1016/j.ajo.2013.03.027. Epub 2013 May 10.]
- ↑ Kim M, Lee J, Park YG, Park YH. Long-Term Analysis of Clinical Features and Treatment Outcomes of Inflammatory Choroidal Neovascularization. Am J Ophthalmol. 2022 Jan;233:18-29. doi: 10.1016/j.ajo.2021.07.014. Epub 2021 Jul 21. PMID: 34298010.
- ↑ [Corolla G. et al. (2021). “Inflammatory Choroidal Neovascular Membranes: Clinical Profile, Treatment Effectiveness, and Visual Prognosis”. J Ophthalmol Vol:2021:9982883. doi: 10.1155/2021/9982883. eCollection 2021.]
- ↑ Jump up to: 15.0 15.1 [Chang L. et al. (Jul 2008). “Bevacizumab treatment for subfoveal choroidal neovascularization from causes other than age-related macular degeneration”. JAMA Ophthalmology, 126(7): pg 941-945. doi: 10.1001/archopht.126.7.941]
- ↑ [Edelman J., Lutz D., Castro M. (Feb 2005). “Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown”. Experimental Eye Research, volume: 80(2):249-58. doi: 10.1016/j.exer.2004.09.013.]
- ↑ [Fine H. et al. (Jan 2009). “Bevacizumab (avastin) and ranibizumab (lucentis) for choroidal neovascularization in multifocal choroiditis”. The Journal of Retinal and Vitreous Diseases, Volume 29(1), pg. 8-12. doi: 10.1097/IAE.0b013e318187aff9]
- ↑ [Invernizzi A. et al. “Twenty-four-month outcomes of inflammatory choroidal neovascularisation treated with intravitreal anti-vascular endothelial growth factors: a comparison between two treatment regimens”. Br J Ophthalmol. 2020 Aug;104(8):1052-1056. doi: 10.1136/bjophthalmol-2019-315257.]
- ↑ [Karti O. et al. (2020). “Inflammatory Choroidal Neovascular Membranes in Patients with Noninfectious Uveitis: The Place of Intravitreal Anti-VEGF Therapy”. Med Hypothesis Discov Innov Ophthalmol, 9(2), pg: 118-126]
- ↑ [Rothova A. (Sept 2002). “Corticosteroids in uveitis”. Ophthalmology Clinics of North America, volume 15(3), pg 389-394, doi: https://doi.org/10.1016/S0896-1549(02)00023-8]
- ↑ Barth T. et al. “Intravitreal anti-VEGF treatment for choroidal neovascularization secondary to traumatic choroidal rupture”. BMC Ophthalmol. 2019; 19: 239. doi: 10.1186/s12886-019-1242-7]

