Cerebral Visual Impairment
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Cortical / Cerebral visual impairment (CVI) is defined as vision loss resulting from damage to the retrogeniculate pathway, in the absence of damage to the anterior afferent visual pathways or ocular structures, or vision loss that is greater than expected for the degree of ocular pathology.[1] CVI occurs due to insults at the level of the cortex, which includes geniculostriate lesions, and the subcortex, which includes focal white matter lesions resulting in periventricular leukomalacia. Delayed visual maturation is thought to be on the spectrum of CVI, resulting from a temporary dysfunction of higher cortical centers. The measurement of visual functions such as visual acuity, contrast sensitivity, and visual field extent should, additionally include, according to the patient’s ability to understand and respond, perceptual visual functions including second-order motion perception, feature detection, visual attention, and memory.
With increased survival of preterm babies and better perinatal care, CVI is now the leading causes of visual impairment in developed countries and increasingly common worldwide.[2] Concomitant causes of visual impairment reported in children with multiple disabilities may be secondary to primary ocular pathology such as uncorrected refractive error, cataract, nystagmus, retinopathy of prematurity (ROP), optic nerve atrophy and delayed visual maturation. The prevalence of CVI in childhood has been steadily rising over the past few decades from a reported incidence of 36 per 100,000 in the late 1980s to 161 per 100,000 in 2003.[1][3]
Disease
The term cortical / cerebral visual impairment (CVI) is preferred over cortical blindness, as the central nervous system remains plastic and the presence of extra-geniculostriate visual pathways precludes total loss of sight, even when there is complete destruction of the striate cortex. Some prefer cerebral visual impairment over cortical visual impairment, owing to the subcortical involvement of the optic radiations in premature infants who are at risk for periventricular leukomalacia.
Recently, a paper by Costa proposed the need for clarification of terms and a proposal of a new term. The new propose term is Central Visual Impairment (CVI) as the Major Diagnostic Term under which we would have Cortical Visual Impairment (termed CoVI) and Cerebral Visual Impairment (termed CeVI). CoVI includes deficits in visual functions exclusively due to lesions in the primary visual pathway, the visual cortex, and associated visual areas. Cerebral Visual Impairment (termed CeVI) when other brain areas such as the cerebellum, brainstem, or thalamus are affected, typically in addition to CoVI. The latter typically includes presence of nystagmus, strabismus, accommodation deficits, and and vestibule-ocular, pupillary impairment[4]. Yet, due to the lack of literature consensus we will continue to refer to this entity interchangeably for now. Regardless of terminology, either cortical or subcortical neurological injury in young children will ultimately affect the cerebral cortex.[5]
Risk Factors
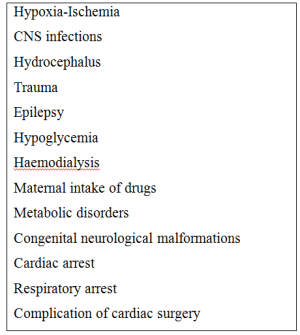
Perinatal or postnatal hypoxic ischemic encephalopathy among term or preterm infants is the most common cause of CVI.[1] [6] Preterm birth, with increased risk of periventricular leukomalacia or intraventricular hemorrhage, is associated with increased chance of developing CVI.[2] CVI is also the most common cause of visual impairment among patients with cerebral palsy.[7] In fact, cerebral palsy is diagnosed in about half of children with CVI.[8] Interestingly, boys may be more affected than girls.[5] Additional risk factors are listed in Table 1.
Pathophysiology
Hypoxia
Hypoxic-ischemic brain injury is, by far, the most common cause of pediatric CVI.[6] The resulting pattern of injury caused by the hypoxic insult, however, is mainly defined by the age at which the insult occurs, and differs greatly between term and pre-term children.
In term infants, the areas between the circulation of anterior and middle cerebral arteries and medial and posterior cerebral arteries are the most typically affected as they are watershed areas of the cerebral cortex. Loss of vascular flow autoregulation induced by hypoxia leads to hypoperfusion of these watershed territories, resulting in infarction of the frontal and parieto-occipital areas. The striate cortex is frequently affected, and occipital visual areas and temporal and parietal cortices, are also commonly involved.
Preterm neonates, on the other hand, rarely suffer parasagittal infarctions from hypoxic-ischemia. More commonly, periventricular deep white matter is involved, especially when the insult occurs earlier, specifically between 24 and 34 weeks of gestation. There is a transient, susceptible watershed zone in the periventricular white matter that later is replaced with the adult vasculature. Long penetrating vessels derived mainly from the middle cerebral arteries run from the pial surface and terminate in the deep periventricular white matter. Active development of this periventricular vasculature occurs during the last 16 weeks of gestation. The number of short penetrators and anastomoses between the long and short penetrators increases in the third trimester with a consequent decrease in vulnerable end zones and border zones. Capillaries in this region are prone to hemorrhage from hypoxic-ischemia.
As a result of hypoxic insult, the germinal matrix produces glial and neuronal cells which migrate eccentrically to populate the cerebrum. Additionally, immature oligodendrocytes and subplate neurons which are present around the ventricles, are more vulnerable to ischemia than mature oligodendrocytes located elsewhere. All of this explains the specific pattern of damage that characterizes periventricular leukomalacia (PVL).
Meningitis
Meningitis and hydrocephalus were considered to be the most common causes of CVI in past.[9] In more recent series, infections account for 11.8% to 15% of cases of CVI.[10] The occipital cortex is more susceptible to damage produced by Haemophilus influenzae, and hence, it is the most common organism causing CVI. Pneumococci, meningococci and herpes simplex virus are other causative agents, which have been implicated to cause ocular and cerebral visual problems.[11] Onset of visual impairment is typically late in the course of the infection, and multiple accompanying neurologic sequelae commonly occur. Different mechanisms by which infection might injure the brain include thrombophlebitis, arterial occlusion, hypoxic-ischemic damage, venous sinus thrombosis and hydrocephalus.
Hydrocephalus
Hydrocephalus affects vision by causing optic atrophy through various mechanisms, and can also affect posterior visual pathways which run close to lateral ventricles. A combination of anterior and posterior visual involvement is frequent. Although ventricular dilatation can occlude the posterior cerebral arteries, chronic distention of the posterior cortex is a frequent mechanism by which hydrocephalus causes CVI. It is well known that shunt malfunction can cause CVI, but paradoxically, rapid correction of raised intracranial pressure by shunting can also occasionally cause CVI. Additionally, high intracranial pressure caused by untreated hydrocephalus can cause secondary optic atrophy, compounding any associated CVI.
Trauma
Head trauma is another cause of pediatric CVI (approximately 4% of cases as reported in two studies).[12] Damage may be transient or permanent. Shaken baby syndrome is a common cause of post-traumatic CVI. Transient vision loss in children can also occur after trivial injuries, and may be accompanied by headache, confusion, drowsiness, vomiting, and seizures.
Epilepsy
Epilepsy, chiefly, infantile spasms can result in central visual impairment. The specific pathogenesis of visual impairment in patients who have infantile spasms is unknown. Furthermore, some treatments for seizures can temporarily compound vision issues and cause diplopia and oscillopsia, while one specific medication for infantile spasms, Vigabatrin, has been associated with peripheral visual loss secondary to retinal toxicity.[13][14]
Other
Congenital brain malformations (lissencephaly, schizencephaly, holoprosencephaly), metabolic and neurodegenerative disease, hypoglycemia, hemodialysis, cerebrovascular accidents, and brain tumors are among other reported causes of CVI.[2] There is implication of underlying genetic factors that may be playing a role. [15]
Diagnosis
Ocular features:
Afferent system
- Cognitive visual function may be impaired in most of these children leading to misinterpretation regarding what is in the visual field, and where things are visuospatially. Visual impairment in CVI can be as severe as no light perception to normal visual acuity. Vernier acuity has been found to be more affected than grating acuity.[16] Vision among patients with CVI may improve under conditions of low luminence.[17] Visual field defects can be difficult to assess in children with CVI due to frequent developmental delays. However, constricted fields and "swiss cheese" fields, or hemianopic defects are the most common.[1] Decreased contrast sensitivity, photophobia, and even paradoxic light gazing are also common.[2] They may have difficulty recognizing faces (proposagnosia) and difficulty recognizing more than one object at a time (simultanagnosia).[5]
- Optic atrophy is common and can be secondary either to hypoxia involving the optic nerves or papilledema, as in cases of hydrocephalus. In a study by V. Khetpal et al, optic atrophy was found to be a feature in 40% patients of cerebral visual loss.[12]
Efferent system
Esotropia or exotropia is common among patients with CVI.[10] Infantile exotropia is more common than esotropia in patients with cerebral visual loss. However, children with PVL tend to present with esotropia, which should be differentiated from infantile esotropia. Dyskinetic strabismus, where esotropia can transform to exotropia on a momentary basis and tends towards exotropia over time, is more common among patients with cerebral palsy[18] and may also be associated with CVI. Consequently, repeated evaluation and frequent deviation measurements are therefore crucial before planning any surgical intervention for observed ocular misalignment. Nystagmus, present in 11% of patients in one study, is surprising given that nystagmus is typically associated with causes in the anterior visual pathway. In the case of CVI, this likely indicates some intact striate cortex, as patients are not typically completely blind.[10] Nystagmus may also indicate concomitant optic nerve or retinal pathology.
Systemic features
CVI is often associated with other neurologic deficits including cerebral palsy, developmental delay, hemiparesis, microcephaly, hearing problems, behavioural problems, myelomeningocele, progressive degenerative disorders, and hypotonia. Some patients may also have superimposed anterior afferent pathology from optic nerve–related disease, see above. Severely visually impaired children often have various blind mannerisms like rocking, head banging, head flopping, eye pressing, light gazing, thumb sucking, and finger-flicking.
Diagnostic procedures
Diagnosis of CVI is of utmost importance in a child with evidence of low vision in the setting of a normal structural ocular examination. However, suspicion and education should begin in the immediate neonatal period, when risk factors can be readily identified.
Visual Evoked Response (VER)
In the past, investigators heavily relied on findings of VER and EEG for a diagnosis of cerebral visual loss. However, a normal flash VER recording can be obtained even in patients with cerebral visual loss, as results are mediated by the extra-geniculostriate visual system.
Electroencephalography (EEG)
EEG was previously considered a valuable diagnostic tool, classically showing multiple eleptiform waves originating from the occipital lobe. However, due to the availability of high-resolution imaging, the role of EEG and VER in making a diagnosis of CVI has lessened in recent years.
Neuroimaging
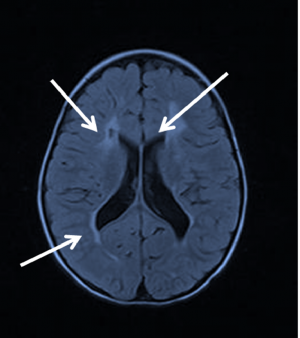
CT and MRI help to differentiate between the degree of possible optic nerve involvement, and suspected cortical involvement, and can help provide clues about prognosis and visual recovery. The extent and location of brain damage is important. MRI is always recommended in children with low APGAR scores.[19]
The presence of acute brain injury seen on brain MRI attributed to hypoxic-ischemia is now considered a significant feature of CVI, as per a recent task force on neonatal encephalopathy. Severity of visual impairment may be predicted by the clinical severity of HIE shown at birth. The pattern of lesions on MRI can be broadly classified into 3 categories: periventricular leukomalacia, diffuse cerebral atrophy, and multi-cystic encephalopathy.
Children with periventricular leukamalacia, encephalic cyst and diffuse cerebral atrophy are much less likely to have significant gains in visual function, while children with mild damage on MRI are more likely to have a better prognosis.
Management
While there is no evidence-based treatment at this time, prevention of preterm birth and HIE may also help prevent CVI. Cooling may reduce death and disabilities associated with hypoxic ischemic encephalopathy.[20]
Management of children with CVI requires a combined and coordinated effort between the ophthalmologist, neurologist and rehabilitation services. Some degree of visual recovery is seen in the vast majority of children with cerebral visual impairment, although the improvement tends to be gradual over months, and the exact mechanism is unclear. Lambert et al have summarized various theories proposed for the visual improvement and suggest that the initial insult producing cerebral visual impairment may not cause cellular death but rather just interrupt the normal protein synthesis of neurons, thereby causing a delay in myelination, dendrite formation and synaptogenesis.[21]
It has now been postulated that improvement of sight in patients with CVI is actually a form of delayed visual maturation.
Any concomitant ophthalmic disorders must be treated, including underlying amblyopia and refractive error. Strabismus and motility evaluations can be difficult, especially in the setting of variable strabismus, so a careful approach is necessary prior to proceeding with any surgical intervention. Nonetheless, as early esotropia is more detrimental to visual development than exotropia, large, manifest esodeviations are an indication for early surgery. Under-correction by 15-20% is usually planned in order to avoid consecutive exotropia over long term follow up, as over-correction is common in this population.[22]
The problems in patients with cerebral visual loss are not limited to vision, but are complex. A multidisciplinary approach is thus necessary not just for diagnosis but also for management.
Rehabilitation
Although most children with CVI show some improvement overtime, 90% remain visually handicapped and should qualify for rehabilitation services.[10] Although visual stimulation is encouraged, no studies at this time have demonstrated improvement with visual stimulation that is significantly greater than spontaneous improvement.[2]
Rehabilitative support must include an approach that addresses the psychosocial impact on patients and their families. Light reflex stimulation is a therapy performed in a completely dark and quiet room, whereby a flashlight is shined briefly in each eye, pausing 5 seconds between eyes, for 1 min and repeated 30 times per day. The ability for perceiving an object's outline can be developed by shining a penlight onto a target in a totally darkened room for 1 minute. Simple distinct shapes like circles, triangles, or stars on white cardboard with a black image or black cardboard with a white image shown 10 times a day, can help children to identify shapes.[23]
To address deficits such as simultanagnosia, a simplified environment with minimal patterns and high contrast colors may improve recognition; near vision may be better than distance vision with fewer distractions, and double spacing may be useful for children who can read.[2] Lower levels of ambient light may be useful.[17] With deficits such as proposagnosia, having family members or caregivers wear the same bright colors may improve recognition.[2] Additional time may be needed to complete tasks, and classroom positioning to account for any identifiable visual field defects may also be helpful.
Each child with CVI is likely to have their own unique visual and motor deficits, necessitating a truly individualized approach.
References
- ↑ 1.0 1.1 1.2 1.3 Good WV, Jan JE, deSa L, Barkovitch AJ, Groenveld M, Hoyt CS. Cortical visual impairment in children: a major review. Surv Ophthalmol 1994;38:351-64.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Chang MY, Borchert MS. Advances in the evaluation and management of cortical/cerebral visual impairment in children. Surv Ophthalmol. 2020 Nov-Dec;65(6):708-724
- ↑ Hoyt CS. Visual function in the brain-damaged child. Eye 2003;17: 369-84.
- ↑ Marcelo Fernandes Costa. Cerebral versus cortical visual impairment: eliminating the conflict and renewing the terminology. .BMC ophthalmol. 2024 May 16;24:213. doi: 10.1186/s12886-024-03469-8. PMCID: PMC11097435 PMID: 38755573
- ↑ 5.0 5.1 5.2 Good WV. Cortical visual impairment: new directions. Optom Vis Sci. 2009 Jun;86(6):663-5.
- ↑ 6.0 6.1 Matsuba CA, Jan JE. Long-term outcome of children with cortical visual impairment. Dev Med Child Neurol. 2006; 48: 508 –512
- ↑ Duke R, Eyong K, Burton K, MacLeod D, Dutton GN, Gilbert C, Bowman R. The effect of visual support strategies on the quality of life of children with cerebral palsy and cerebral visual impairment/perceptual visual dysfunction in Nigeria: study protocol for a randomized controlled trial. Trials. 2019 Jul 10;20(1):417.
- ↑ West MR, Borchert MS, Chang MY. Ophthalmologic characteristics and outcomes of children with cortical visual impairment and cerebral palsy. J AAPOS. 2021 Aug;25(4):223.e1-223.e6.
- ↑ Hoyt CS. Hydrocephalus, brain anomalies and cortical visual impairment. In: Taylor D, Hoyt CS, eds. Pediatric Ophthalmology and Strabismus. Philadelphia, Pa: Elsevier Saunders; 2005: 675– 686
- ↑ 10.0 10.1 10.2 10.3 Huo R, Burden SK, Hoyt CS, Good WV. Chronic cortical visual impairment in children: aetiology, prognosis, and associated neurological deficits. Br J Ophthalmol. 1999 Jun;83(6):670-5.
- ↑ El Azazi M, Malm G, Forsgren M. Late ophthalmologic manifestations of neonatal herpes simplex virus infection. Am J Ophthalmol. 1990;109:1–7
- ↑ 12.0 12.1 Khetpal V, Donahue SP. Cortical visual impairment: etiology, associated findings, and prognosis in a tertiary care setting. J AAPOS. 2007;11:235–239
- ↑ Remler BF, Leigh RJ, Osorio I, Tomsak RL. The characteristics and mechanisms of visual disturbance associated with anticonvulsant therapy. Neurology. 1990;40:791–796
- ↑ "Vigabatrin: The Problem of Monitoring for Peripheral Vision Loss in Children". American Association for Pediatric Ophthalmology and Strabismus. July 2017. https://aapos.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=781a381d-6dae-ede4-bcf7-a3db9973bf15
- ↑ Emogene Shaw, Ian Flitcroft, Richard Bowman, Kate Baker, Cerebral visual impairment: genetic diagnoses and phenotypic associations. J Med Genet. Genomics England Research Consortium. 2024 Mar 8;61(6):605–612. doi: 10.1136/jmg-2023-109670 PMCID: PMC11137471 PMID: 38458753
- ↑ Skoczenski AM, Good WV. Vernier acuity is selectively affected in infants and children with cortical visual impairment. Dev Med Child Neurol. 2004;46:526–32.
- ↑ 17.0 17.1 Good WV, Hou C. Sweep visual evoked potential grating acuity thresholds paradoxically improve in low-luminance conditions in children with cortical visual impairment. Invest Ophthalmol Vis Sci. 2006;47:3220–4
- ↑ Buckley E, Seaber JH. Dyskinetic strabismus as a sign of cerebral palsy. Am J Ophthalmol. 1981 May;91(5):652-7.
- ↑ ACOG. Executive summary: neonatal encephalopathy and neurological outcome. Obstet Gynecol.2014:123:896-901
- ↑ Shankaran, Seetha, et al. "Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy." New England Journal of Medicine 353.15 (2005): 1574-1584.
- ↑ Lambert SR, Hoyt CS, Jan JE, Barkovich J, Flodmark O. Visual recovery from hypoxic cortical blindness during childhood. Computed tomographic and magnetic resonance imaging predictors. Arch Ophthalmol. 1987 Oct; 105(10):1371-7.
- ↑ Swaminathan M, Shah SV, Mittal S, Gunasekaran A. Results of bilateral medial rectus recession for comitant esotropia in patients with developmental delay. Strabismus 2014; 22:138-42.
- ↑ Myers G, Leisman G. Rehabilitation of cortical visual impairment in children Int J Neurosci. 2006 Sep;116(9):1015-33.