Central Toxic Keratopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Central toxic keratopathy (CTK) describes a rare, acute, non-inflammatory complication most associated with excimer laser ablation surgery (e.g., laser assisted in situ keratomileusis (LASIK) or photorefractive keratopathy (PTK) surgery). However, idiopathic cases have been reported[1] and an association with contact lens use has been made[2][3][4]. Potential cases have been associated with selective laser trabeculoplasty[5] and topical anesthetic[6] use as well. Fraenkel and colleagues first described CTK in 1989 as being inflammatory in nature but the condition has subsequently been recognized as a non-inflammatory process.
Etiology
Although the exact cause of CTK is ambiguous [7][8], several theories abound regarding possible factors contributing to the development of CTK. Among the most prominent suggestions in the literature are photoactivation of povidone-iodine by the excimer laser, laser-induced keratocyte apoptosis of corneal matrix [8], intraoperative exposure to meibomian gland secretions [8], marking pen ink [9], talc from latex surgical gloves [7], or post-surgical debris from the microkeratome blade [7].
Pathophysiology
Pathophysiologic changes in CTK are incompletely understood; however, some aspects of the disease have been established. One principal aspect of the disease is corneal thinning due to stromal loss. The most well -supported theory to explain the underlying mechanism is an apoptosis of keratinocytes. This mass keratinocyte apoptosis may also be the cause of the observed central opacity[3][10][8][11][12]. Tissue loss is most prominent in the first month, and results in a loss of anterior tangential curvature; while posterior tangential curvature remains relatively unaltered[11]. An alternative theory to the observed thinning proposes that micro edema into the corneal periphery causes mild stretching[13]. Following the thinning, the corneal thickness begins to increase over a matter of weeks to months. This aspect of the disease is less studied, but may be the result of stromal remodeling or epithelial wound healing[10].
Physical Examination
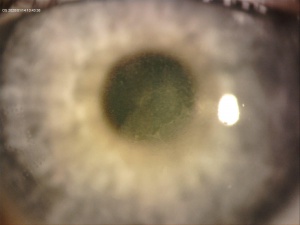
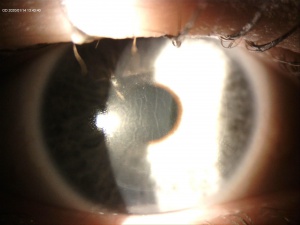
A central opacity in the corneal stroma should be evident on exam (Fig. 1), and may be associated with striae (Fig. 2). Inflammation should not be readily apparent. A hyperopic shift and resultant decrease in visual acuity is also a common feature of the disease. OCT imaging may show the opacity as an “inverse dome” that extends from the anterior to posterior stroma with homogenous reflectivity. Confocal microscopy has revealed activated keratinocytes and potential Descemet’s membrane folding[2][7][8][11][14][15].
Diagnosis
The characteristic clinical appearance in CTK bears a striking resemblance to a number of inflammatory and infectious conditions including contact lens-induced keratitis [4][16]
, infectious keratitis[4], post-photorefractive keratectomy (PRK) haze[9], epithelial ingrowth, diffuse lamellar keratitis (DLK) [7][8], and corneal haze secondary to increased intraocular pressure (pressure-induced stromal keratitis, or PISK)[9].
Despite its shared features with other disease processes, CTK can be appropriately distinguished from masquerading conditions via a thorough history and an awareness of timing of onset, existing inciting factors, and by the nature of clinical findings that each disease characteristically exhibits.
For example, although both DLK and CTK both lead to a reduction in corneal clarity and a hyperopic shift, the post-surgical opacification seen in DLK tends to be nonlocalized [7] and subepithelial [9] while the post-surgical haze in CTK is centralized and extends anteriorly or posteriorly from the interface. Similarly, unlike infectious or inflammatory processes which exhibit characteristic features, CTK rarely presents with anterior chamber reaction, conjunctival hyperemia, or ciliary flush [7].
Scheimpflug imaging and optical coherence tomography (OCT) imaging studies may offer diagnostic clues into CTK-specific corneal alterations. Specifically, Scheimpflug imaging is useful in highlighting the exaggerated flattening of the anterior cornea that commonly accompanies CTK [7]. Similarly, OCT images of eyes affected with CTK will typically demonstrate central corneal thinning, central backscattering, and higher internal reflectivity when compared to normal eyes [7].
Signs
Patients with CTK typically develop dense central corneal opacification, stromal tissue loss, striae, and significant hyperopic refractive shift [7][8]. These signs typically begin on post-operative day 2-6. The central corneal opacification in CTK may begin with diffuse lamellar keratitis (DLK) on post-surgery day 1 or 2 and quickly gives rise to a dense opacification of the central corneal stroma [8]; however prior DLK is not necessary for diagnosis and cases have been reported without preceding DLK[8]. This corneal opacity and hyperopic shift characteristically persists for 2-18 months before spontaneously resolving to a degree [7].
Symptoms
Common symptoms associated with CTK include photophobia, pain, floaters, redness, halos, and a hyperopic shift. CTK often leads to reduced best spectacle-corrected visual acuity (BSCVA) in affected patients [7][8].
Differential Diagnosis
- Diffuse lamellar keratitis (DLK)
- Infectious keratitis
- Interface fluid
- Corneal haze secondary to increased IOP (Pressure-Induced Stromal Keratitis or PISK)
- Superficial punctuate keratitis
- Epithelial ingrowth
- Trauma
Management and Follow up
Since the centralized stromal haze in CTK spontaneously resolves within 18 months without treatment, close monitoring and regularly-scheduled follow-up remains the primary management strategy in patients with CTK [7][9]. Although corticosteroids were used in the past, the recent discovery that CTK is non-inflammatory in nature coupled with the fact that CTK is unresponsive to steroid therapy has discouraged the use of these medications in the management of CTK[7][9].
Additional medical therapies that may be beneficial include metalloprotease inhibitors (such as doxycycline) and agents that promote corneal wound healing (such as ascorbic acid); whereas agents, such as mitomycin-c, that are known to disrupt these processes may worsen outcomes in patients with CTK[17][18][19][20]. Finally, based on the micro edema hypothesis of corneal flattening, hyperosmotic agents have been tried by some with beneficial effects[13].
As stromal tissue loss is a prominent feature of the disease, surgical management is controversial due to fear of further thinning the cornea. However, some authors have argued for the use of more invasive procedures (e.g., flap lift and irrigation) in the treatment of CTK, indicating that additional stromal loss is not inevitable[21]. One study showed less corneal flattening and thinning and better visual acuity in patients who underwent surgical management than non-surgically managed patients[22].
Complications
Given its rarity and the fact that it shares striking similarities with other infectious and inflammatory processes, CTK is understandably frequently misdiagnosed and difficult to manage. CTK typically resolves spontaneously within 18 months with minimal complications or permanent sequelae [7][8]. Most complications, however, that arise in the setting of CTK typically occur as a result of mismanagement with steroids or other similar medications that can exacerbate preexisting refractive or anatomic alterations in this patient population [7].
Prognosis
The prognosis for CTK may be fair to good. In most cases, the condition resolves within 18 months of the inciting event; however, hyperopic visual defects may remain.
References
- ↑ Wagh VK, De Benito-Llopis L, O’Brart DPS. Idiopathic stromal keratitis resembling central toxic keratopathy. J Cataract Refract Surg. 2015. doi:10.1016/j.jcrs.2015.06.001
- ↑ Jump up to: 2.0 2.1 Ting DSJ, Ghosh S. Central Toxic Keratopathy after Contact Lens Wear and Mechanical Debridement: Clinical Characteristics, and Visual and Corneal Tomographic Outcomes. Eye Contact Lens. 2019. doi:10.1097/ICL.0000000000000575
- ↑ Jump up to: 3.0 3.1 Hsu M, Tu E, Bouchard C. Confocal microscopy of contact lens keratitis presenting as central toxic keratopathy. Eye Contact Lens. 2011. doi:10.1097/ICL.0b013e31821c0401
- ↑ Jump up to: 4.0 4.1 4.2 Moshirfar M, Kurz C, Ghajarnia M. Contact lens-induced keratitis resembling central toxic keratopathy syndrome. Cornea. 2009. doi:10.1097/ICO.0b013e318197ec3a
- ↑ Liu ET, Seery LS, Arosemena A, Lamba T, Chaya CJ. Corneal edema and keratitis following selective laser trabeculoplasty. Am J Ophthalmol Case Reports. 2017. doi:10.1016/j.ajoc.2016.11.007
- ↑ Chen HT, Chen KH, Hsu WM. Toxic keratopathy associated with abuse of low-dose anesthetic: A case report. Cornea. 2004. doi:10.1097/01.ico.0000114127.63670.06
- ↑ Jump up to: 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 Moshirfar M, Hazin R, Khalifa YM. Central Toxic Keratopahy after LASIK surgery. Current Opinion in Ophthalmology 2010 Jul;21(4):274-9.
- ↑ Jump up to: 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 Sonmez B, Maloney RK.Central toxic keratopathy: description of a syndrome in laser refractive surgery. Am J Ophthalmol 2007;143:420-7.
- ↑ Jump up to: 9.0 9.1 9.2 9.3 9.4 9.5 Hazin R, Daoud YJ, Khalifa YM. What is Central Toxic Keratopathy Syndrome if it is not Diffuse lamellar Keratitis Grade IV? Middle East Afr J Ophthalmol. 2010 Jan;17(1):60-2.
- ↑ Jump up to: 10.0 10.1 Moshirfar M, Hazin R, Khalifa YM. Central toxic keratopathy. Curr Opin Ophthalmol. 2010. doi:10.1097/ICU.0b013e32833a8cb2
- ↑ Jump up to: 11.0 11.1 11.2 Sikder S, Khalifa YM, Neuffer MC, Moshirfar M. Tomographic corneal profile analysis of central toxic keratopathy after LASIK. Cornea. 2012. doi:10.1097/ICO.0b013e31821de341
- ↑ Hau SCH, Allan BD. In vivo confocal microscopy findings in central toxic keratopathy. J Cataract Refract Surg. 2012. doi:10.1016/j.jcrs.2012.01.010
- ↑ Jump up to: 13.0 13.1 Morgan LA. Central toxic keratopathy: a case study and literature review. Optometry. 2012.
- ↑ Thornton IL, Foulks GN, Eiferman RA. Confocal microscopy of central toxic keratopathy. Cornea. 2012. doi:10.1097/ICO.0b013e3181f7f109
- ↑ Bühren J, Baumeister M, Cichocki M, Kohnen T. Confocal microscopic characteristics of stage 1 to 4 diffuse lamellar keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2002. doi:10.1016/S0886-3350(02)01307-X
- ↑ Moshirfar M, Madsen M, Wolsey D. Re: central toxic keratopathy: description of a syndrome in laser refractive surgery. Am J Ophthalmol 2007;144:332; author reply 333-4.
- ↑ Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001. doi:10.1006/exer.2001.1054
- ↑ Smith VA, Cook SD. Doxycycline - A role in ocular surface repair. Br J Ophthalmol. 2004. doi:10.1136/bjo.2003.025551
- ↑ Chen J, Lan J, Liu D, et al. Ascorbic acid promotes the stemness of corneal epithelial stem/progenitor cells and accelerates epithelial wound healing in the cornea. Stem Cells Transl Med. 2017. doi:10.1002/sctm.16-0441
- ↑ Blanco-Mezquita T, Espandar L, Torres R, et al. Does mitomycin C cause toxicity in the cornea after photorefractive keratectomy? A comparative wound-healing study in a refractive surgery animal model. Cornea. 2014. doi:10.1097/ICO.0000000000000219
- ↑ Tu KL, Aslanides IM. Surgical intervention in central toxic keratopathy. Eur J Ophthalmol. 2013.
- ↑ Moshirfar M, MacGregor N, West W, McCabe S, Miller C, West D, Shmunes K, Hoopes P. Five-year occurrence and management of central toxic keratopathy after femtosecond laser-assisted LASIK. J Refract Surg 2021;37(1):25-31.