Cataract Surgery with Bag-In-the-Lens (BIL) fixation technique
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Surgical Therapy
Cataract surgery or lens replacement surgery remains one of the most performed procedures in ophthalmology. Worldwide, more than 9.5 million cataract surgeries are performed each year[1]. The gold standard surgical procedure consists of lens phacoemulsification and insertion of a permanent artificial intraocular lens in the bag[2]. In 20 to 30 %[3][4], a posterior capsular opacification (called "after-cataract") appears due to the proliferation and migration of retained lens epithelial cells across the posterior capsule. This complication is treated by a posterior capsulotomy with Nd-YAG laser. Like any surgical procedure, there are some risks[5]: sudden increase in intraocular pressure[6], retinal tear[7], retinal detachment[7], macular edema[8], impacts on the artificial lens, dislocation of the artificial lens[9], iris hemorrhage, corneal edema, or optical aberrations[10].
Background
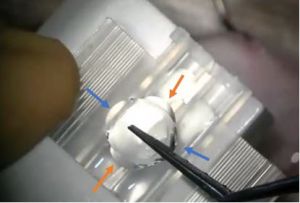
Bag-In-the-Lens (BIL) fixation technique was described by Tassignon et al. in adults in 2002[11] and in pediatric cataracts in 2007[12]. Bag-In-the-Lens intraocular lens (BIL IOL) (Morcher₢ 89A, FCI, Germany)(Video 1) is a biconvex acrylic hydrophilic intraocular lens with a new design composed of a 5 mm diameter round optical and two perpendicular haptics, forming an interhaptic groove with a total diameter of 7.5 mm . It is the most common BIL used and exists in spheric monofocal and toric. Soon, a multifocal BIL will be marketed.
BIL IOL Morcher₢ Type 89D is intended for children with an axial length inferior to 18 mm; or with a limbal diameter of 8-9mm. BIL IOL Morcher₢ Type 89D is a smaller intraocular lens with an optic of 4.5 mm-diameter and a total diameter of 6.5 mm. Finally, BIL IOL Morcher₢ Type 89F has a larger anterior haptic with a total diameter of 8.5mm and is intended for combined cataract and posterior vitrectomy.
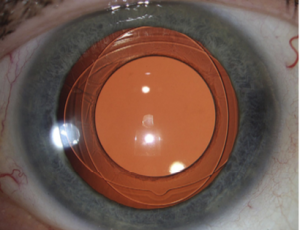
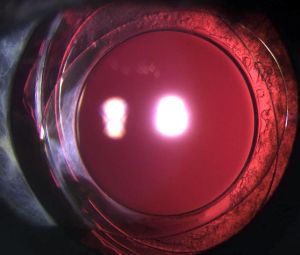
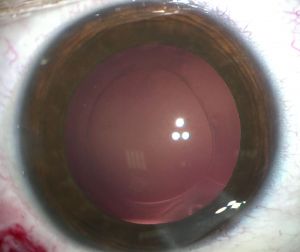
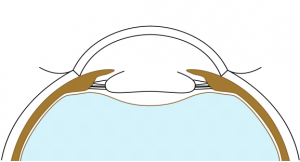
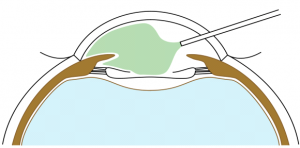
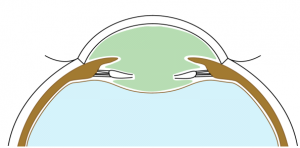
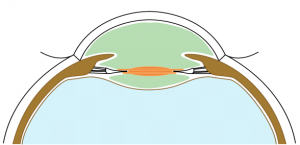
Anterior and posterior capsulorhexes of equal diameter are performed and then captured into the groove of a specific IOL (Figure 1). Thus, the term "Bag-in-the-Lens" has the opposite concept to the currently used "lens-in-the-bag". BIL blocks the two capsulorhexes in its groove, and the two capsules, anterior and posterior, merge all around, forming a new barrier to epithelial cells (Figures 2 and 3). The perfect overlap of the two capsulorhexes by 360° decreases the risk of posterior capsule opacification.
These results have been shown in vitro and first in vivo on human donor eyes and rabbits eyes[13] by Pr Tassignon, who first published in 2002 the results in 10 eyes[11]; including a bilateral congenital cataract in a 4-year-old child. In 2007, Tassignon and al.[12] confirmed that this technique is safe and kept a clear visual axis in babies and children. In 2010, Werner et al.[14] collected postmortem results of 6 eyes of 4 patients, confirming that any proliferative material remains confined to the intercapsular space, sparing the visual axis.
Since then, several studies of several countries confirmed that BIL is safe, feasible, and particularly interesting in pediatric cataracts[15][16][17].
Indications
This technique is particularly interesting for patients that cannot undergo Nd-YAG laser (pediatric cataracts, patients operated under general anesthesia such as disabled persons or patients with dementia), have a history of chronic intraocular inflammation, or in which a clear visual axis is necessary for the follow-up (diabetic patients, retinal diseases).
But this technique can be preferred in all cases.
Contraindications
There are few contraindications to this type of implant, namely traumatic cataracts with large capsular effraction, severe microphthalmia, severe zonulopathy or subluxated lens.
Surgical Technique
• Description of BIL technique in adults (Video 2)
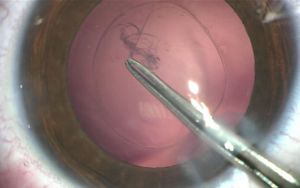
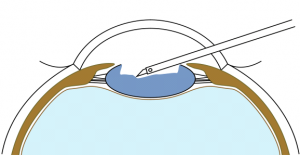
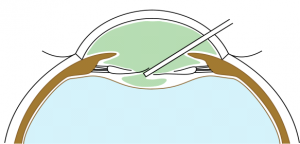
The main difference of BIL technique is the need to realize an anterior and posterior capsulorhexes of equal 5mm diameter. To perform the anterior capsulorhexis, a 5.2 mm-diameter PolyMethylMetAcrylate (PMMA) ring caliper (Morcher₢, FCI, Germany) is inserted. It is removed right after having completed the capsulorhexis with the Troutman clam. Callisto₢ can also be used. Phacoemulsification and masses aspiration are done as usual. Viscoelastic is injected in the sulcus, on the top of the remaining anterior capsule to join both capsules, and not inside the capsular bag. A micropuncture of the posterior capsule is performed with a 30-gauge needle. Viscoelastic product (for example, Viscoat₢) is injected through the hole in the Berger space to push back the anterior vitreous membrane. Then, a posterior continuous curvilinear capsulorhexis is done, of the same size as the anterior one (Figure 4).
Stackable capsulorhexes are obtained (Figure 5).
An anterior vitrectomy is seldom necessary, only if there is vitreous in the anterior chamber.
BIL is not a preloaded intraocular lens. It must be put in the cartridge with dispersive viscoelastic, paying attention to inserting the posterior haptic first, which should be directed under the capsulorhexes (Figure 6). BIL is injected into the anterior chamber. The posterior haptic is gently pushed behind the posterior capsule at the 6 o'clock position until both anterior and posterior capsules are engaged in the implant. The insertion of the BIL is completed doing gentle right to left movements until the insertion between both capsules is in 360 degrees. After aspiration of the viscoelastic product, the injection of a myotic agent is necessary to avoid iris capture. Intracamerular injection of antibiotic is done as usual.
| Phacoemulsification as usual | |
| After phacoemulsification: calibrated anterior capsulorhexis of 5mm diameter | |
| Injection of viscoelastic product on the top of the remaining anterior capsule (not in the bag) | |
| No viscoelastic in the bag | |
| Puncture of the posterior capsule with a 30-gauge needle | |
| Injection of dispersive viscoelastic in the hole to push back the anterior vitreous face and the Berger Space | |
| Viscoelastic is injected into the Berger space and on the top of the remaining anterior capsule; both anterior and posterior capsules are joined | |
| Realization of the posterior continuous curvilinear capsulorhexis, of equal diameter, using the anterior one as a caliper | |
| Injection of BIL in the anterior chamber | |
| Insertion of BIL in both capsules over 360 degrees |
• BIL Tips and tricks in adults
The ring caliper inserted in the anterior chamber is helpful to calibrate the anterior capsulorhexis. Nevertheless, it does not avoid a capsulorhexis radial tear. The ring must be centered on the Purkinje reflects. Check regularly that the ring does not move. Be sure that the ring is well supported against the anterior capsule and not in suspension in the anterior chamber. Aim to have a slightly smaller capsulorhexis by staying INSIDE the caliper. It is better to have a smaller capsulorhexis than an oversized one that doesn't permit BIL anymore. As mentioned above, Calisto₢ (with a 5.2 mm projection) can be used instead of the ring caliper.
In case of poor pupil dilatation, be careful using the Malyugin₢ ring with BIL. Indeed, the total diameter of BIL is 7.5mm, while the total Malyugin₢ ring diameter is 6.25mm. Therefore, you must remove the Malyugin₢ ring before inserting BIL. If there is too much myosis to ensure correct visualization and BIL insertion, iris hooks are easier to use.
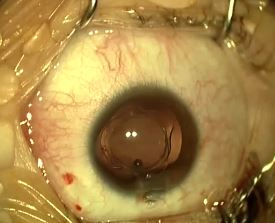
After inserting BIL in the anterior chamber, sometimes the haptics can stick together, making it impossible to insert BIL in between the capsules. The haptics are not round anymore, but wavy. (Figure 7 ). In that case, using a clamp and the micromanipulator, you will gently push one haptic, holding the other one, to create some space in between and have the normal BIL(Video 3). Then, you will be able to fix it into both capsules again.
In case of a significant defect in zonular support, bean-shaped rings have been created[18]. In addition to a capsular tension ring, bean-shaped segments expand the BIL technique in large zonular defects (for example, in pseudoexfoliation or traumatic cataracts). The outer rings of the segments provide tension on the capsule, supplementing the capsular tension ring, while the inner tension ring supports and centers the BIL[19].
In case of combined cataract with posterior segment surgery using tamponade agents, there is a risk of iris capture. The tamponade agent may push the BIL up and the iris may pass under the haptics and get blocked. To avoid this risk, use the type Morcher₢ 89F which has a larger anterior haptic to avoid iris incarceration. If a Morcher₢ Type 89A is used, do not dilate the pupil until at least 50% of the agent is absorbed (depending on the gas used, one week with SF6, two weeks with C2F6, and three to four weeks with C3F8). In case of iris capture due to an involuntary pupil dilatation, ask the patient to keep his/her head in a face down for 1-2 hours and then use pilocarpine.
• Description of the BIL technique in children (Video 4)
Pediatric cataract surgery is more challenging because of the usual myosis and the elasticity of the capsule, which makes the capsulorhexis difficult to carry out. It requires a skilled and trained surgeon. Surgery is done under general anesthesia.
The main difference between adult and children is the use of a 4.5mm diameter PMMA ring caliper (until four to five years-old) to anticipate the elasticity of the capsules. After five, we use the 5.2 mm diameter ring caliper, also used in adults.
Otherwise, surgical steps are the same.
• BIL Tips and tricks in children
For children with an axial length under 18mm or with limbal diameter of 8-9mm, use the type Morcher₢ 89D. This BIL has a smaller size, specific for this population. Calisto₢ is not used in children, because of the cornea's curvature that decrease the quality of reflex, and increase the risk of having an oversized capsulorhexis. Use the 4.5mm caliper under 3-4 years old. After 3-4 years old, use the normal 5.2 mm caliper or Calisto₢.
We recommend that you train on adults before children, because BIL has a learning curve.
Anterior vitrectomy is seldom used with BIL technique, only if vitreous is coming through the anterior chamber. Nevertheless, it is more often with children, especially in unilateral cataract and vitreo-lenticular interface anomalies (persistence of fetal vascular system)[20]. In case of the hyaloid canal (Cloquet's canal), it is possible to cut it out without performing anterior vitrectomy (video 5).
In children, there is a myopic shift and lens power calculation remains unpredictable. It has been demonstrated that the predictive error is correlated with age: the younger the child is, the higher the residual refractive error[21]. The post-operatory refractive result in 87 eyes with BIL was satisfactory[22], and one of the advantages of BIL is the possibility of changing implants without excessive trauma, as seen in video 6.
Outcomes
• In adults :
BIL technique is remarkable because there is no visual axis opacification (VAO) if BIL is well fixed in both anterior and posterior capsules. In 2006, De Groot et al.[23] reported 63 eyes with BIL implantation and at least one year of follow-up. There was no lens epithelial cells (LEC) proliferation on the visual axis during the follow-up (the mean follow-up was of 22.7 months, ranging from 12 to 64 months); LEC proliferation was confined to the peripheral capsular bag. The technique was safe and feasible in sixty of 63 eyes (95%). A conventional IOL was necessary in two cases. In one eye, postoperative luxation of BIL IOL into the vitreous occurred due to oversized anterior and posterior capsulorhexes. Three eyes had early postoperative iris incarceration in the lens groove that required surgery.
Inflammation occurs frequently after surgery and is usually treated by dexamethasone drops 3 times a day for one month. BIL technique showed a low rate of inflammatory response[24]. Lower inflammation is probably due to epithelial cell block between the two capsules, no friction of the ciliary body or iris by the haptics, intact anterior hyaloid membrane, no anterior vitrectomy, and hydrophilic implant. Auchere et al.[25] found that BIL helps avoid posterior synechiae of the iris in phaco-vitrectomy (1 case, 2%) compared to lens-in-the-bag implantation (22 cases, 40%, p<0.001).
Cataract surgery using the BIL implantation technique in diabetes patients has comparable quality visual outcomes to the standard lens-in-the-bag techniques. In a study of BIL in diabetic patients[26], diabetic retinopathy progression occurred in 3 of 54 eyes, with no statistically significant difference in diabetic retinopathy grading. Three cases of clinically significant macular edema were detected over the one-year follow-up period. Because BIL prevented posterior capsule opacification in 100% of cases (mean follow-up of one year), fundus for further diabetic monitoring and treatment was simplified.
The risk of retinal detachment (RD) after cataract surgery was comparable to the lens-in-the-bag technique. Over 1323 eyes, 19 RD occurred (1.44%). The 1-year RD incidence was 0.49% (5 RD cases in 1024 eyes) (0.00% in patients without risk factors). The 2-year cumulative RD incidence was 0.84% (9 RD cases in 931 eyes: 0.15% without risk factors). Four statistically significant risk factors were identified: male gender, young age at the time of surgery (<60 years), axial myopia (axial length ≥25 mm), and history of contralateral RD[27].
During a 7-year follow-up, Tassignon et al.[24] enrolled 807 eyes of 547 patients; 326 of the eyes (40.40%) had ocular comorbidity. In the 481 eyes without ocular comorbidity, the mean decimal corrected distance visual acuity was 0.276 logMAR (logarithmic minimum angle resolution) preoperatively and 0.012 logMAR postoperatively. The mean postoperative achieved spherical equivalent was 0.48 ± 0.83 diopters (D), and the mean targeted refraction -0.24 ± 0.71 D. The A-constant was modified from 118.4 to 118.04. Posterior capsule opacification (PCO) did not occur in any adult eye during the follow-up. Retinal detachment after BIL IOL implantation occurred in 10 eyes (1.24%). In 19 eyes(2.4%), the iris was captured by the IOL haptics postoperatively. Hypopyon occurred in 3 patients and toxic anterior segment syndrom in 1 patient.
A cohort of disabled patients was performed in France[28], including 60 eyes of 36 subjects. Ten patients had Down syndrome, five had cerebral palsy, six autism, two psychiatric troubles, and one with deep deafness. Two groups were observed, one with BIL implantation (36 eyes), and the other without BIL implantation (22 eyes). BIL tended to decrease the rate of reintervention in disabled patients (8% versus 14%, p=0.6594). There was more anterior luxation in BIL group, perhaps because of more eye-rubbing in disabled patients.
• In children :
The end of the pediatric cataract surgery is only the beginning of a long amblyopia reeducation requiring the parents' acceptance and involvement. A clear visual axis is fundamental to a successful reeducation. BIL technique is exciting in children because it avoids visual axis opafication (VAO). First results in children in 2007 showed this technique was safe and feasible[12]. A 5-year follow-up study confirmed that in 91.2%, a clear visual axis was maintained. Four cases of VAO in 46 eyes (8.6%) were reported, all due to a wrong positioning of the lens[29]. This result was confirmed by Nystrom et al. in 2018 (4.6%)[15], Lytvynchnuk et al. in 2020 (5.6%)[16], Bailleul in 2020 (5.2%)[30], and Boonstra et al. in 2021(8%)[17]. VAO rate is much lower than in studies with standard implants.
In our serie, we had one case of visual axis opacification due to a traumatic BIL anterior. The haptics didn't block the lens epithelial cells anymore and proliferation occured. Here is the video (Video 7) of the reintervention.
In literature, the prevalence of posterior capsule opacification varies from 10 to 100% depending on the surgical technique studied. If the posterior capsule remains intact, 100% secondary cataract is observed, as in Vasavada study[31] and 95.8% in Knight study[32]. Performing a posterior capsulotomy and anterior vitrectomy during pediatric cataract surgery lowered the rate of VAO. The posterior capsule opacification (PCO) rate is estimated at 15.6% in the Ram study[33], 10.8% in the prospective Vasavada study[34], up to 70% reported by Lundvall[35] in 2006.
Secondary glaucoma remains a threatening complication because of possible vision loss. After BIL implantation, the rate of secondary glaucoma was low and confirmed in several studies : 2.2% in Looveren[29] study; 7.8% in Lytvynchnuk[16] study, 1.3% in Bailleul[30] study and 0% in Boonstra[17] study. In comparison, after lens-in-the-bag implantation, Freedman reported a 17% risk of developing secondary glaucoma in children aged 1 to 6 months with five years of follow-up after cataract surgery[36], 17% also for Mataftsi with 4.3 years follow-up[37]. Magnusson reports 12% glaucoma in Swedish children followed for 9.7 years[38]. This small secondary glaucoma incidence with BIL technique is inconsistent with Nystrom et al., who found 13.8% of secondary glaucoma with 2.9 years of follow-up[15][39]. Nevertheless, Nystrom reported an earlier age of surgery (under 12 weeks), more frequent posterior tamponade, several comorbidities, and more frequent posterior vitrectomies.
From a physio-pathological point of view, several risk factors explain secondary glaucoma. First, age at the time of the surgery and the size of the eye (corneal diameter and axial length) are evocated. Indeed, the size of the haptics of the implants could damage the trabeculum in small eyes. BIL IOL geometry aims to be physiological, with a round optic and small haptics. The total diameter of BIL Morcher₢ 89A is 7.5mm versus 13mm for Alcon Acrysoft SN60WF₢ or Bausch and Lomb EyeceeOne₢ implant. Second, the barrier between the anterior and posterior segment is crucial and seems protective against glaucoma[37]. Hyaloid membrane impedes vitreous invasion and trabeculum meshwork damage. We hypothesize that anterior vitrectomy, commonly used to prevent VAO, destroys this barrier. With BIL technique, anterior vitrectomy is seldom used (9.2% in our study[30]). Third, intraocular inflammation leads to the use of steroids and consequently to increased intraocular pressure[40]. There is less inflammation after BIL technique than the standard implants, probably thanks to the collapse of the capsule avoiding epithelial cells to activate the inflammatory cascade. In summary, several explanations are possible for this low rate: small size and specific geometry, quasi-physiological location in the eye separating the anterior and posterior segments, no systematic prior vitrectomy, less inflammatory response, and limited use of steroids.
Concerning the refractive and functional results, Nystrom[15] reported a spherical equivalent of -0.3 +/- 2.8 D. Van Looveren[29], with 5-year follow-up, finds a myopic average spherical equivalent with an average of -1.9 +/- 3.7 D. Lytvynchnuk[22] made a study about the predictive error in children cataract surgery with BIL and found that it was correlated with age. The risk of refractive errors increases with earlier surgery age. One advantage of the BIL is the possibility of relatively easy implant exchange in case of refractive error. (Video 6)
Here is a summary of the main results about pediatric cataract surgery in children with BIL technique in literature :
| Study | Journal | Year | Design | Location | Mean follow-up (years) | Maximal follow-up (years) | Number of surgeons | Number of eyes | Number of eyes <12 months | Number of eyes<6 months | Number of peroperative vitrectomy | VAO | Clear visual axis (%) | Glaucoma | Iris capture | Other complications | Number of global reinterventions | Number of reintervention needed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tassignon[12] | JCRS | 2007 | Retrospective | Antwerp
Belgium |
1.45 | 5.6 | 1 (MJT) | 34 | 7 | 4 | 1 (2.9%) | 3 (8.8%)
All surgical |
91.2% | 2 (5.8%)
(medical) |
1 | 2 IOL luxations | 6 (17.6%) | 1 |
| Van Looveren[29] | JCRS | 2015 | Prospective | Antwerp
Belgium |
6.5 | 13 | 1 (MJT) | 46 | 11 | 8 | 4 (8.6%) | 4 (8.6%)
All surgical |
91.3% | 1 (2.2%)
(surgical) |
0 | 2 lysis anterior synechiaes | 7 (15.2%) | 1 |
| Nyström | Acta
Ophtalm |
2018
2019 |
Retrospective | Gothenburg
Sweden |
2.8 | 5.8 | 1 (AN) | 109 | 29 | ?
24 <6w |
? | 5 (4.6%)
All surgical |
95.4% | 15 (13.8%)
7: 1 surgery 2: >2 surgeries 7 medical |
0 | 8 IOL luxations
3 IOL exchange 2 IOL explant |
28 (25.6%) | >2 for 2 patients with secondary glaucoma |
| Lytvynchnuk[16] | Acta
Ophtalm |
2020 | Retrospective | Gissen
Germany |
? | 1 | 2
(WS, LL) Never both |
90 | 31 | ? | 26 (28.9%) | 5 (5.6%)
All surgical |
94.4% | 7 (7.8%)
5 medical 2 surgical |
2 | 1 IOL luxation
2 synechiaes |
12/90 (13.3%) | 1 |
| Boonstra[17] | Acta
Ophtalm |
2021 | Retrospective | Bergen
Norway |
3 | 4.85 | 1 (NEB) | 50 | 6 | ? | 1 (2%) | 4 (8%)
1 surgical |
92 | 0 (0%)
1 high IOP cortico steroid inducted |
2 | 2 bleb revisions (trab before cataract surgery) | 5 (10%) | 1 |
| Bailleul[30] | ESCRS | 2021 | Retrospective | Caen
France |
3.27 | 9.4 | 3
(CB, EB, ALL) together |
76 | 17 | 9 | 7 (9.2%) | 4 (5.2%)
All surgical |
94.8% | 1 (1.3%)
medical after post vitrectomy |
2 | 1 IOL luxation
2 Retinal detachments |
9 (11.8%) | 1 |
Specific Complications
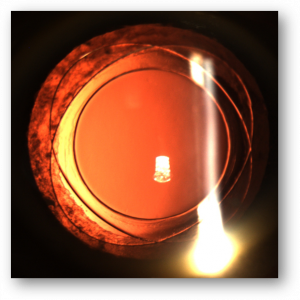
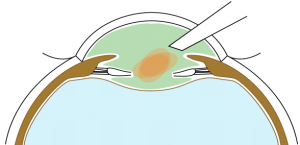
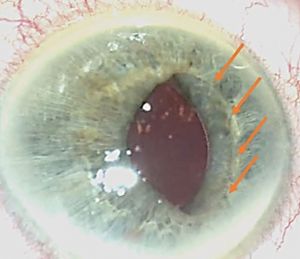
Iris capture is a specific complication because of the geometry of the implant. When the iris tightens, it can pass under one of the haptics. It does not cause vision loss but causes the pupil ovalization, giving a "cat's eye" appearance (Figure 8). This complication is avoided immediately postoperatively by injecting a myotic agent at the end of the intervention. The two cases mentioned in our series appeared several months after the surgery. Pharmacologic pupil dilation is usually sufficient to release the iris. If not, a quick surgery is performed to reposition the implant. It is vital to inform the parents so that they detect this complication.
One case of opacified BIL was described in 2015[41] due to calcium and phosphate deposit. BIL was exchanged without further complications.
Capsular pseudoexfoliation can appear on BIL (Figure 9) without visual consequences.
Conclusion
In conclusion, BIL technique is a valid alternative for cataract surgery with less visual axis opacification, less secondary glaucoma, and less inflammatory response. It is particularly interesting in the pediatric population.
References
- ↑ Foster A, Frcophth F, Farmer J, Stevens S. Vision 2020 : the Right To Sight Vision 2020 : the Cataract Challenge. Community Eye Heal. 2000;13(34):17-19.
- ↑ Davis G. The Evolution of Cataract Surgery. Mo Med. 2016;113(1):58-62.
- ↑ Milazzo S, Grenot M, Benzerroug M. La cataracte secondaire. J Fr Ophtalmol. 2014;37(10):825-830.
- ↑ Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology. 1998;105(7):1213-1221.
- ↑ Billotte C, Berdeaux G. Adverse clinical consequences of neodymium:YAG laser treatment of posterior capsule opacification. J Cataract Refract Surg. 2004;30(10):2064-2071.
- ↑ Stark WJ, Worthen D, Holladay JT, Murray G. Neodymium:YAG Lasers: An FDA Report. Ophthalmology. 1985;92(2):209-212.
- ↑ 7.0 7.1 Pattern P, Kingdom U. Retinal breaks and detachment after neodymium : YAG laser posterior capsulotomy Is LASEK really possible in the rabbit eye ? Hyaluronidase allergy after peribulbar anesthesia with orbital inflammation. 2005;31(August):1480-1481.
- ↑ Steinert RF, Puliafito CA, Kumar SR, Dudak SD, Patel S. Cystoid macular edema, retinal detachment, and glaucoma after Nd:YAG laser posterior capsulotomy. Am J Ophthalmol. 1991;112(4):373-380.
- ↑ Framme C, Hoerauf H, Roider J, Laqua H. Delayed intraocular lens dislocation after neodymium:YAG capsulotomy. J Cataract Refract Surg. 1998;24(11):1541-1543.
- ↑ Levy J, Lifshitz T, Klemperer I, et al. The effect of Nd:YAG laser posterior capsulotomy on ocular wave front aberrations. Can J Ophthalmol. 2009;44(5):529-533.
- ↑ 11.0 11.1 Tassignon MJBR, De Groot V, Vrensen GFJM. Bag-in-the-lens implantation of intraocular lenses. J Cataract Refract Surg. 2002;28(7):1182-1188.
- ↑ 12.0 12.1 12.2 12.3 Tassignon MJ, De Veuster I, Godts D, Kosec D, Van den Dooren K, Gobin L. Bag-in-the-lens intraocular lens implantation in the pediatric eye. J Cataract Refract Surg. 2007;33(4):611-617.
- ↑ De Groot V, Tassignon MJBR, Vrensen GFJM. Effect of bag-in-the-lens implantation on posterior capsule opacification in human donor eyes and rabbit eyes. J Cataract Refract Surg. 2005;31(2):398-405.
- ↑ Rozema J, Werner L, Tassignon M-J, Zaugg BE, De Groot V. Clinical and Histopathologic Evaluation of Six Human Eyes Implanted with the Bag-in-the-Lens. Ophthalmology. 2009;117(1):55-62.
- ↑ 15.0 15.1 15.2 15.3 15.4 Nyström A, Almarzouki N, Magnusson G, Zetterberg M. Phacoemulsification and primary implantation with bag-in-the-lens intraocular lens in children with unilateral and bilateral cataract. Acta Ophthalmol. 2018;96(4):364-370.
- ↑ 16.0 16.1 16.2 16.3 Lytvynchuk LM, Thiele M V., Lorenz B. Analysis and management of intraoperative and early postoperative complications of bag-in-the-lens intraocular lens implantation in different age groups of paediatric cataract patients: report of the Giessen Paediatric Cataract Study Group. Acta Ophthalmol. 2020;98(2):e144-e154.
- ↑ 17.0 17.1 17.2 17.3 Boonstra N, Haugen OH. Bag-in-the-lens intraocular lens in paediatric cataract surgery : intraoperative and postoperative outcomes. 2021:1-7.
- ↑ Tassignon M-J, Dhubhghaill SN. Bean-shaped ring segments for capsule stretching and centration of bag-in-the-lens cataract surgery. J Cataract Refract Surg. 2014;40(1):8-12.
- ↑ Ní Dhubhghaill S, Dogaroiu AC, Zakaria N, Tassignon M-J. Modified bean-shaped ring segments for suture fixation of the bag-in-the-lens intraocular implant. J Cataract Refract Surg. 2017;43(8):1003-1006.
- ↑ Van Looveren J, Vael A, Ideler N, Sillen H, Mathysen D, Tassignon MJ. Influence of the vitreolenticular interface in pediatric cataract surgery. J Cataract Refract Surg. 2018;44(10):1203-1210.
- ↑ Neely DE, Plager DA, Borger SM, Golub RL. Accuracy of intraocular lens calculations in infants and children undergoing cataract surgery. J AAPOS. 2005;9(2):160-165.
- ↑ 22.0 22.1 Lytvynchuk LM, Thiele M V., Schmidt W, Lorenz B. Precision of bag-in-the-lens intraocular lens power calculation in different age groups of pediatric cataract patients: Report of the Giessen Pediatric Cataract Study Group. J Cataract Refract Surg. 2019;45(10):1372-1379.
- ↑ De Groot V, Leysen I, Neuhann T, Gobin L, Tassignon MJ. One-year follow-up of bag-in-the-lens intraocular lens implantation in 60 eyes. J Cataract Refract Surg. 2006;32(10):1632-1637.
- ↑ 24.0 24.1 Tassignon MJ, Gobin L, Mathysen D, Van Looveren J, De Groot V. Clinical outcomes of cataract surgery after bag-in-the-lens intraocular lens implantation following ISO standard 11979-7:2006. J Cataract Refract Surg. 2011;37(12):2120-2129.
- ↑ Auchère Lavayssiere C, Lux AL, Beraud G, Degoumois A, Billotte C, Denion É. Bag-in-the-lens implantation helps avoid posterior synechiae of the iris after phacovitrectomy. J Cataract Refract Surg. 2019;45(10):1386-1392.
- ↑ Lauwers N, Ní Dhubhghaill S, Mathysen DGP, Tassignon MJ. Assessment of the bag-in-the-lens implantation technique in diabetic patients. Ophthalmologica. 2013;229(4):212-218.
- ↑ Tassignon MJ, Van Den Heurck JJI, Boven KBM, et al. Incidence of rhegmatogenous retinal detachment after bag-in-the-lens intraocular lens implantation. J Cataract Refract Surg. 2015;41(11):2430-2437.
- ↑ Bailleul H, Billotte C, Lux A-L. Comparaison Entre Implantation Bag-In-the-Lens et Implantation Dans Le Sac Ou Le Sulcus Dans La Chirurgie de La Cataracte de Patients En Situation de Handicap. Soutenance de diplôme de mémoire de spécialité en ophtalmologie, France; 2019.
- ↑ 29.0 29.1 29.2 29.3 Looveren J Van, Dhubhghaill SN, Godts D, Bakker E, Veuster I De, Mathysen DGP. Pediatric bag-in-the-lens intraocular lens implantation : Long-term follow-up. 2015:1685-1692.
- ↑ 30.0 30.1 30.2 30.3 Bailleul H. Rate of re-intervention in paediatric cataract surgery with the bag-in-the-lens fixation: ten years of experience. Poster at the 38th Meeting of ESCRS. 2020.
- ↑ Vasavada A, Chauhan H. Intraocular lens implantation in infants with congenital cataracts. J Cataract Refract Surg. 1994;20(6):592-598.
- ↑ Flitcroft DI, Knight-Nanan D, Bowell R, Lanigan B, O’Keefe M. Intraocular lenses in children: Changes in axial length, corneal curvature, and refraction. Br J Ophthalmol. 1999;83(3):265-269.
- ↑ Ram J, Brar GS, Kaushik S, Gupta A, Gupta A. Role of posterior capsulotomy with vitrectomy and intraocular lens design and material in reducing posterior capsule opacification after pediatric cataract surgery. J Cataract Refract Surg. 2003;29(8):1579-1584.
- ↑ Vasavada AR, Trivedi RH, Nath VC. Visual axis opacification after AcrySof intraocular lens implantation in children. J Cataract Refract Surg. 2004;30(5):1073-1081.
- ↑ Lundvall A, Zetterström C. Primary intraocular lens implantation in infants: Complications and visual results. J Cataract Refract Surg. 2006;32(10):1672-1677.
- ↑ Freedman SF, Lynn MJ, Beck AD, Bothun ED, Örge FH, Lambert SR. Glaucoma-related adverse events in the first 5 years after unilateral cataract removal in the infant aphakia treatment study. JAMA Ophthalmol. 2015;133(8):907-914.
- ↑ 37.0 37.1 Mataftsi A, Haidich AB, Kokkali S, et al. Postoperative glaucoma following infantile cataract surgery: An individual patient data meta-analysis. JAMA Ophthalmol. 2014;132(9):1059-1067.
- ↑ Magnusson G, Abrahamsson M, Sjo J. Glaucoma following congenital cataract surgery : An 18-year longitudinal follow-up. 2000:65-70.
- ↑ 39.0 39.1 Nyström A, Magnusson G, Zetterberg M. Secondary glaucoma and visual outcome after paediatric cataract surgery with primary bag-in-the-lens intraocular lens. Acta Ophthalmol. 2019;(2008):1-9.
- ↑ Kwok AKH, Lam DSC, Ng JSK, Fan DSP, Chew SJ, Tso MOM. Ocular-hypertensive response to topical steroids in children. Ophthalmology. 1997;104(12):2112-2116.
- ↑ Ní Dhubhghaill S, Van Os L, De Keizer RJW, Taal M, Zakaria N, Tassignon M-J. Intraocular lens exchange technique for an opacified bag-in-the-lens. J Cataract Refract Surg. 2015;41(5):924-928.