Cataract Surgery After Vitrectomy/Phacovitrectomy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Vitrectomy is a surgical procedure to remove the vitreous gel in the treatment of posterior segment pathology. The most frequent complication of pars plana vitrectomy (PPV) is cataract formation, particularly nuclear sclerotic cataract. In a large national registry data-based study from the UK that involved more than 2000 eyes with PPV performed for different indications, the risk of cataract surgery following PPV was in the range of 40% and the 1-, 2-, 3-, and 5-year cataract surgery rates were 50, 70, 75, and 85%, respectively.[1][2]Although the exact mechanism of cataract formation or progression after PPV is not clear, with one of the leading theories postulating that increased oxygen tension after PPV causes oxidative damage of crystalline lens proteins and cataract progression. Other theories include altered milieu following vitreous removal, trauma, and iatrogenic causes.[3] Older age, use of tamponade, and complex extended PPV can all be contributing factors to cataract progression after PPV.
While the overall risk of complications is low, there are still post-operative complications that must be considered. Vitrectomy can lead to the formation and accelerated progression of cataracts, most commonly, the nuclear sclerotic (NS) type[4]. A meta-analysis of 51 studies found that the incidence of post-vitrectomy cataract varies considerably from 6-100%[5]. This wide range can be attributed to ocular pathology initially requiring vitrectomy, variable follow up intervals, and the subjective grading of cataracts.
Concerns for post-PPV complications have prompted ophthalmologists to seek alternative surgical techniques to treat various ocular pathologies while minimizing the risk of cataract formation[6]. This has led to the practice of phacovitrectomy that involves the combined or sequential performance of phacoemulsification and vitrectomy[7]. Because cataract formation is the most common complication after vitreoretinal surgery, prophylactic removal of the lens provides numerous benefits for the patient which include improved post-surgical VA and reduced recovery time. Rishi et al. evaluated long-term postoperative outcomes between patients receiving combined or sequentially performed phacoemulsification and vitrectomy. In that study, there was no significant difference in visual outcome between the two groups; however, both groups were reported to have improved postoperative VA with a mean logMAR of 0.63 ± 0.89 (P < .05) in the combined group and mean logMAR 0.57 ± 0.60 (P < .05) in the sequential group following the last visit (at 10 years). 32% of the eyes in the sequential surgery group had an observed progression of cataracts at 5.7±7.7 months[7].
The formation of cataracts associated with vitrectomy are associated with multiple risk factors perioperatively. In turn, post-vitrectomized eyes requiring cataract surgery are associated with higher rates of complications compared to cataract management alone.[8][4][9].
Pre-operative considerations
Before proceeding with cataract surgery, a thorough review of the patient’s ocular history should be obtained, including onset of symptoms, prior surgeries, history of trauma, and history of intravitreal injections. Significant risk factors that must be considered pre-operatively include age and a preexisting cataract. NS cataracts rarely form in patients younger than 50 years old. In a study by Melberg et al., PPV with gas tamponade was found to result in cataract formation in 7% of patients younger than 50, compared to 80% in patients older than 50 at two-year follow-up[8]. The differences in cataract progression between patients above and below 50 years old was also found to be significantly accelerated in the older group[10].
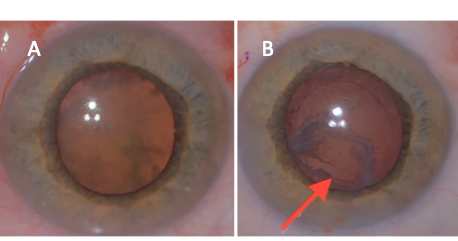
Of note, if a patient develops a white cataract shortly after vitrectomy or injection, the surgeon should consider the high likelihood of iatrogenic lens capsule violation and consider referring to a vitreoretinal specialist. Even if a cataract forms gradually after vitrectomy, consider the possibility of peripheral posterior capsule injury in cases with significant posterior subcapsular cataract (Figure 1).
A slit lamp examination should be performed with close examination of the posterior capsule for previous lens touch during PPV, and evaluation for signs of phacodenesis and zonular instability. Outpouching or linear irregularities of the posterior capsule may signify previous capsular trauma at the time of PPV. Occasionally, B-scan ultrasonography can be helpful to visualize the posterior capsule when direct visualization is difficult.
Similar to non vitrectomized eyes, optical biometry was found to be more accurate than ultrasound biometry. If a cataract is too dense for optical biometry, immersion ultrasound can be utilized. The surgeon should also be aware that intraocular lens (IOL) calculations can be slightly less accurate due to axial length discrepancies.[11] There is evidence of systematic myopic shift induced in phacovitrectomy eyes for the widely used validated vergence based formulas (Barrett UII, SRK/T, Holladay, Hoffer Q, and Haigis).[12] These formulas have good refractive outcomes in phacovitrectomy; although, the Kane formula, a modern IOL lens power calculation formula, was found to have the highest percentage of eyes within + 0.25 diopters of aimed post-operative refraction.[12] In silicone-filled eyes, IOL-selection can be even more challenging. Modern formulas such as Barrett UII, Hill-RBF, and Kane have been shown to have higher predictability of postoperative refraction with a lower variance of error.[13] Additionally, it has recently been found that eyes that have had prior vitrectomy are at greater risk of IOL-tilt and decentration.[14]
Multifocal lenses and other optically advanced IOL selections should be evaluated with extreme caution due to the risk of IOL-tilt and decentration.[14] In eyes where PPV was done for macula off retinal detachment, it is important to ensure that the axial length measurement was undertaken after the retina was attached and not prior. This is to avoid false reading resulting from the retinal detachment. All patients should be counseled regarding surgical expectations.
Intra-operative considerations and techniques
The vitreous provides posterior support during cataract surgery, and this is critical in maintaining a normal anterior chamber depth (ACD). Eyes that have undergone prior vitrectomy may have an exaggerated ACD due to the lack of vitreous support. Lowering the infusion pressure can help normalize the ACD. Additionally, a retrobulbar or sub-tenon’s anesthetic block may provide posterior pressure for support.
When deciding on wound construction, a short tunnel corneal incision may be beneficial since a long corneal tunnel can result in instruments more vertically oriented causing corneal distortion. Proper wound construction will also aid with less wound leakage during surgery.
The phenomenon of iris-diaphragm retropulsion syndrome (LIRDS), or reverse pupillary block, is especially common in vitrectomized eyes. Due to the laxity of the iris-lens diaphragm, the pupil border becomes stuck against the anterior capsule, and the phaco infusion becomes trapped in front of the iris, which dramatically increases anterior chamber pressure and depth. The pupil will also appear widely dilated (Figure 2A). LIRDS increases the risk of intraoperative aqueous misdirection syndrome, pupillary constriction, and post-operative inflammation.[15] The LIRDS can make surgical maneuvers difficult, and in cases under topical anesthesia, may also be painful for the patient. To relieve this situation, the iris may be lifted off the capsule with the chopper or another blunt instrument to allow fluid equilibrium between the anterior and posterior chambers.[15][16] Maintaining the fluidics and a stable anterior chamber is crucial. Lower infusion pressure, properly constructed wounds, and gentle handling of surgical instruments with care of not removing instruments quickly, can help maintain a stable ACD. Unfortunately, after reverse pupillary block occurs during surgery, the pupil often constricts when it is reversed (Figure 2B).
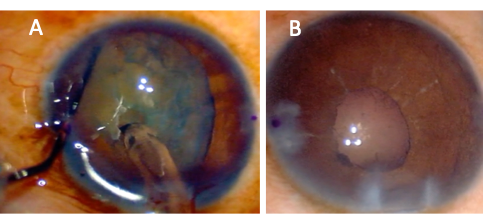
The capsulorrhexis is one of the most important steps in phacoemulsification, and it can be more challenging in post-vitrectomized eyes due to the exaggerated ACD and the lack of posterior support of the lens. More pressure on the anterior lens capsule is typically needed to initiate a capsular flap due to the anatomically decreased resistance. Trypan blue can be considered in cases of a poor red reflex, although the surgeon should be aware that the dye can change the elastic properties of the capsule.[17] If the surgeon is having difficulty initiating a capsulorrhexis flap, then there should be concern for occult zonular dialysis. The difficulty in initiating the flap in these cases is caused by a backward movement of the lens due to the lack of zonular counterpressure or excessive lens movement during the progress of the capsulorrhexis. Capsular striae can be seen when attempting to initiate the capsulorrhexis flap in these cases.[18]
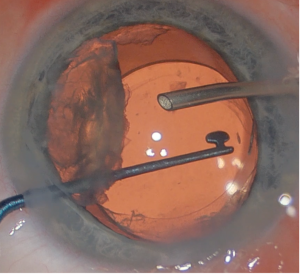
Hydrodissection can be performed in uncomplicated cases. If there is concern from preoperative or intraoperative examination of posterior lens trauma or touch, hydrodissection should be avoided. Instead, hydrodelineation and viscodissection techniques are preferred. For nucleus removal, chopping techniques may be better suited for these cases due to the exaggerated ACD. The surgeon should also be prepared for the possibility of needing a capsular tension ring or segment if there is significant zonular compromise. If there is a posterior capsular rupture from cataract surgery or prior vitrectomy, the lens should be brought anterior out of the capsular bag with viscoelastic material placement. A lens scaffolding technique may be employed to lower the risk of dropped lens fragments (Figure 3). If a patient requires intravitreal injection at time of cataract surgery, for example to manage a coexistent cystoid macular edema, then it is best given before removing viscoelastic material with the eye being firm to avoid inadvertent retinal injury.
Additional intra-operative risk factors may include excess light exposure and inadvertent lens trauma or contact. The eye has natural defense mechanisms which protect it from photo-oxidative damage. Removal of the vitreous would theoretically lead to the breakdown of one of these protective mechanisms and result in oxidative stress on the photo-sensitive lens[19]. Several studies have additionally hypothesized that the prolonged exposure to light energy from the operating microscope or fiberoptic probe may induce lens damage through mechanical disruption, increased intraocular temperatures, or a combination of both[3][4][20]. The risk of “lens touch” during an intraocular procedure has been associated with the rapid development of cataracts postoperatively. In a large study of 1400 phakic eyes undergoing intraocular surgery, 3.7% had an inadvertent lens touch with 94% of these resulting in cataract development. 45% of these were the NS type, 33% PSC, 16% mixed type, and 6% white cataract[21]. While PPV is a risk factor for the formation and progress of NS cataracts, the length of the surgery itself does not appear to have an appreciable effect. Within a sub-cohort of the Vitrectomy for Macular Hole Study, there was no observed difference in NS or posterior subcapsular (PSC) cataract formation and progression in surgeries above or below one hour in duration[3][19].
Post-operative considerations
Careful complete removal of viscoelastic material is recommended to avoid acute post-operative intraocular pressure spike. It can be difficult to achieve complete removal of under the IOL if there are capsular or zonular problems. In these cases, prophylactic oral acetazolamide should be considered and IOP has to monitored in the early postoperative period.
Although post-vitrectomized eyes have a higher risk and necessitate careful planning, cataract surgery in these eyes generally leads to good visual outcomes. About two-thirds of post-vitrectomized eyes that undergo cataract surgery attain 20/40 vision or better.[2] Visual potential from cataract surgery in post-vitrectomized eyes is also dependent on severity of vitreoretinal pathology and baseline vision.[2] There is an increased risk of zonular dialysis in post-vitrectomy cataract surgery compared to routine cataract surgery by approximately 2-fold (1.3% vs. 0.6%).[2] There is a mixed literature on whether prior PPV surgery increases the risk of posterior capsule rupture. Cole and colleagues found a high rate of posterior capsular rupture (12.5%) in a series of 72 eyes.[22] However, our group found no difference in the rates of posterior capsular rupture (1.49% vs. 1.72%) in a large study of 138,754 eyes.[2]
Standard post operative care following a PPV often requires a medium with which to tamponade. Commonly used agents include silicone oil, air, and inert gases such as sulfur hexafluoride and perfluoro propane. Several studies have found that a significant proportion of phakic eyes in contact with a silicone oil tamponade eventually developed a cataract[23][24][25]. The underlying pathophysiology behind the cataractogenic properties of the oil tamponade is unclear. Initial studies have suggested that the oil interferes with the metabolic exchange across the posterior capsule, leading to atrophy and opacification of the lens. Some recent studies have speculated that the silicone oil may increase the permeability of the lens capsule through alterations in the molecular charge barrier. This could lead to anterior lenticular cell edema and apoptosis, resulting in the characteristic fibrous pseudo-metaplasia of the silicone oil cataract[23][24].
Surgical Management
Anesthesia
Most cataract surgeries are performed on an outpatient basis with a combination of topical, regional, block, or sub-Tenon’s infusion anesthetic and monitored anesthesia care (MAC)[26].
General anesthesia is typically reserved for patients who are unable to tolerate local anesthetic options (e.g., significant anxiety, cognitive impairment, history of complications in the fellow eye) with early discontinuation once surgery is completed to avoid elevated intraocular pressure[26][27].
Surgical Techniques
Cataract extractions alone are typically 10-20 minutes in length by an experienced ophthalmologist depending upon technique and clinical complexity of the patient. In patients with a history of vitrectomy, cataract removal has been reported to be more difficult due to a variety of factors including the presence of posterior synechiae, intraoperative miosis, and fluctuations in the fluid dynamics of the eye[5]. Review studies have noted that two factors likely contribute to the additional surgical difficulties associated with post-vitrectomy cataract surgery. The first is that the nucleus of the post-vitrectomy cataract often has a higher density than its age-related counterpart, often requiring increased surgical times and the use of phacodynamic energy. The second was a reported increase in posterior capsule mobility and rupture due to the loss of vitreous in the posterior segment[26].
Phacoemulsification
This is the most utilized technique in high-resource countries for cataract removal.
Phacoemulsification is typically preferred to other methods of extraction as the relative risk of operative complications are lower with a smaller incision. Additionally, patients typically have a faster recovery time and more favorable outcomes[26][5][4].
This method is preferred for the removal of cataracts regardless of the history of PPV[5][19]. Surgical challenges include the presence of posterior synechiae and pupillary changes such as intraoperative miosis, which have been reported in approximately 7% of postoperative PPV eyes[19][28]. To safely perform phaco, surgeons may lyse the posterior synechiae and dilate the pupil manually with iris hooks[28]. Additionally, the changes in the fluid dynamics of the eye leads to anterior chamber depth fluctuations. Avoidance of intraoperative complications related to these changes involves following a set of recommended surgical parameters including a low phaco probe power (30% reduction), low infusion rate (20-25cc/min), and a low infusion bottle height (80cm)[5][19][28].
Extracapsular Cataract Extraction
Extracapsular cataract extraction (ECCE) involves the removal of the intact lens nucleus through a single large incision. The cortex is aspirated, leaving the lens capsule in place. The capsule acts as a sling to support the IOL implant[29].
This technique is typically performed in settings where phacoemulsification is unavailable, not possible (i.e., an extremely dense lens), or in low resource settings[5][19]. Vitrectomized eyes tend to have deeper anterior chambers, leading to a difficult extraction of the sclerotic nucleus of the lens. This is likely due to the loss of vitreous support, posterior segment collapse, and hypotony[19].
Femtosecond laser assisted cataract surgery (FLACS)
The FLACS technique utilizes lasers to assist in the creation of cleavage planes with real-time intraoperative imaging[5][30]. It is thought that the procedure may provide greater precision compared to the manual performance of surgical techniques such as the capsulorrhexis. The technique also reduces the effective phaco energy and time for nuclear fragmentation[30]. While these advantages are attractive, in the setting of a post vitrectomized eye, there appears to be little evidence of benefit in selecting FLACS over the traditional phaco technique[31][10]. Additionally, FLACS may be cost prohibitive for many patients with little advantage over traditional methods[19][30].
Post-operative Complications
Patients who develop or have progression of cataracts following vitrectomy have the option of elective cataract surgery. Despite removing the cataract, some patients may continue to have poor VA due to their baseline retinal pathology. Defining the success of the intervention is relative to the initial state of the patient and may or may not improve VA. As with any ocular surgery, there is a risk of infection (endophthalmitis), inflammation, and bleeding[5]. A few of the post operative complications that appear to be closely associated with cataract surgery post-PPV include cystoid macular edema (CME), retinal detachment, and posterior capsular opacities[26][32][33][34].
The development of CME is theorized to occur because of the increased susceptibility of vitrectomized eyes to the toxokinetics of intravitreal antibiotics and the changes in hydrostatic pressures in the eye[33]. This complication may be prevented with peri-operative topical anti-inflammatory or steroid drugs[33][19]. The prognosis is favorable as most post-PPV CME resolves within a few weeks[35].
Retinal detachment has been reported in approximately 2-8% of vitrectomized eyes following cataract surgery[26][34]. A study of 100 post-PPV eyes found that four presented with a retinal detachment following phaco[34]. Notably, all the eyes with retinal detachment after phaco had the initial PPV performed as a part of the management of previous retinal detachment. Another contributing factor to consider may be the increased traction on the retina due to removal of the vitreous fluid. The careful examination of the retina via ocular imaging prior to cataract surgery, especially in the setting of a previous retinal detachment, is recommended[26][34].
Posterior capsular opacities form after cataract surgery at a greater rate in post-PPV eyes[26][32]. This is seen more often a few weeks after PPV. The use of gas or silicone oil tamponade after PPV is also associated with an increased risk of a permanent posterior capsular opacity[26][32]. There are currently no preventative measures, but the traditional management of posterior capsular opacities may be followed by way of YAG laser treatment[36].
Prognosis
The prognosis of a post-vitrectomy patient following cataract surgery is variable and dependent on the underlying retinal pathology. Overall, most patients had an improvement in VA[26][37][2]. In a study performed by Soliman et al., the outcomes of vitrectomized versus non-vitrectomized (control group) eyes after phacoemulsification cataract removal noted an improvement in VA. Additionally, eyes with a history of vitrectomy had a worse mean postoperative vision of 0.2 logMAR units along with an increased rate of postoperative zonular dialysis and dropped nuclear fragments[2].
Conclusions
As the indications and utilization of PPV continues to rise, it is important to be aware of the possible complications associated with the procedure. PPV is a known risk factor for the progression and development of cataracts. Cataract surgery is generally more difficult in the setting of previous PPV due to changes in ocular anatomy, altered fluid dynamics, and inflammatory changes. The decision to proceed with surgical intervention is made through careful evaluation by the physician, considering the specific clinical presentation. A shared decision-making process with the patient is essential in making an informed selection of surgical technique.
References
- ↑ Jackson TL, Donachie PHJ, Sparrow JM, Johnston RL. United Kingdom National Ophthalmology Database Study of Vitreoretinal Surgery: Report 1; Case mix, complications, and cataract. Eye (Basingstoke) 2013;27.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Soliman MK, Hardin JS, Jawed F, et al. A Database Study of Visual Outcomes and Intraoperative Complications of Postvitrectomy Cataract Surgery. Ophthalmology 2018;125.
- ↑ Jump up to: 3.0 3.1 3.2 Feng H, Aadelman RA. Cataract formation following vitreoretinal procedures. Clinical Ophthalmology (Auckland, NZ) 2014;8:1957.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Panozzo G, Parolini B. Cataracts associated with posterior segment surgery. Ophthalmology Clinics of North America 2004;17(4):557‐68.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Do DV, Gichuhi S, Vedula SS, Hawkins BS. Surgery for postvitrectomy cataract. Cochrane Database of Systematic Reviews 2018, 10;1(1):CD006366. doi: 10.1002/14651858.CD006366.pub4.
- ↑ Barca, F., & Rizzo, S. Combined Phacoemulsification and Pars Plana Vitrectomy. Retina Surgery Global Perspectives. 2012. https://retinatoday.com/articles/2012-may/combined-phacoemulsification-and-pars-plana-vitrectomy
- ↑ Jump up to: 7.0 7.1 Rishi P, Hariprasad SM, Rishi E. Long-Term Outcomes of Combined Phacoemulsification and Pars Plana Vitrectomy Surgery. Ophthalmic Surg Lasers Imaging Retina. 2021 Sep;52(9):470-477. doi: 10.3928/23258160-20210824-01. Epub 2021 Sep 1. PMID: 34505807.
- ↑ Jump up to: 8.0 8.1 Melberg NS, Thomas MA. Nuclear sclerotic cataract after vitrectomy in patients under 50 years of age. Ophthalmology 1995;102(10):1466‐71.
- ↑ Thompson JT. The role of patient age and intraocular gas use in cataract progression after vitrectomy for macular holes and epiretinal membranes. Am J Ophthalmol. 2004;137(2):250–257.doi: 10.1016/j.ajo.2003.09.020.
- ↑ Jump up to: 10.0 10.1 Submacular Surgery Trials Research Group. Surgical removal vs observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic: I. Ophthalmic findings from a randomized clinical trial: Submacular Surgery Trials (SST) Group H Trial: SST Report No. 9. Archives of Ophthalmology 2004;122(11):1597‐611.
- ↑ Esteban O, Mateo J, Casas P, et al. Cataract Surgery in Post-Vitrectomized Eyes. In: Current Cataract Surgical Techniques.; 2021.
- ↑ Jump up to: 12.0 12.1 Hipólito-Fernandes D, Elisa Luís M, Maleita D, et al. Intraocular lens power calculation formulas accuracy in combined phacovitrectomy: an 8-formulas comparison study. International Journal of Retina and Vitreous 2021;7:1–8.
- ↑ Lwowski C, Miraka K, Müller M, et al. IOL calculation using eight formulas in silicone oil filled eyes undergoing silicone oil removal and phacoemulsification after retinal detachment. American Journal of Ophthalmology 2022;0.
- ↑ Jump up to: 14.0 14.1 Tan X, Liu Z, Chen X, et al. Characteristics and Risk Factors of Intraocular Lens Tilt and Decentration of Phacoemulsification After Pars Plana Vitrectomy. Translational Vision Science & Technology 2021;10.
- ↑ Jump up to: 15.0 15.1 Ghosh S, Best K, Steel DHW. Lens-iris diaphragm retropulsion syndrome during phacoemulsification in vitrectomized eyes. Journal of Cataract and Refractive Surgery 2013;39.
- ↑ Cionni RJ, Barros MG, Osher RH. Management of lens-iris diaphragm retropulsion syndrome during phacoemulsification. Journal of Cataract and Refractive Surgery 2004;30.
- ↑ Jardeleza MSR, Daly MK, Kaufman JD, et al. Effect of trypan blue staining on the elastic modulus of anterior lens capsules of diabetic and nondiabetic patients. Journal of Cataract and Refractive Surgery 2009;35.
- ↑ Yaguchi S, Yaguchi S, Yagi-Yaguchi Y, et al. Objective classification of zonular weakness based on lens movement at the start of capsulorhexis. PLoS ONE 2017;12.
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 19.8 Hernandez-Bogantes E, Abdala-Figuerola A, Olivo-Payne A, Quiros F & Wu L Cataract Following Pars Plana Vitrectomy: A Review, Seminars in Ophthalmology. 2021; 36:8, 824-831, DOI: 10.1080/08820538.2021.1924799
- ↑ Libre PE. Intraoperative light toxicity: a possible explanation for the association between cataract surgery and age-related macular degeneration. Am J Ophthalmol. 2003;136(5):961. doi:10.1016/S0002-9394(03)00906-1.
- ↑ Elhousseini Z, Lee E, Williamson TH. Incidence of lens touch during pars plana vitrectomy and outcomes from subsequent cataract surgery. Retina. 2016;36(4):825–829. doi:10.1097/IAE.0000000000000779.
- ↑ Cole CJ, Charteris DG. Cataract extraction after retinal detachment repair by vitrectomy: Visual outcome and complications. Eye 2009;23.
- ↑ Jump up to: 23.0 23.1 Casswell AG, Gregor ZJ. Silicone oil removal. I. The effect on the complications of silicone oil. Br J Ophthalmol. 1987;71 (12):893–897. doi:10.1136/bjo.71.12.893.
- ↑ Jump up to: 24.0 24.1 Gonvers M. Temporary silicone oil tamponade in the management of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1985;100(2):239–245. doi:10.1016/0002-9394(85) 90788-3.
- ↑ McCuen BW 2nd, De Juan E Jr., Landers MB 3rd, Machemer R. Silicone oil in vitreoretinal surgery. Part 2: results and complications. Retina. 1985;5(4):198–205. doi:10.1097/00006982-198500540-00002.
- ↑ Jump up to: 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 26.9 Biró Z, Kovacs B. Results of cataract surgery in previously vitrectomized eyes. J Cataract Refract Surg. 2002; 28(6):1003-6. doi: 10.1016/s0886-3350(02)01237-3.
- ↑ Seidenari P, Santin G, Milani P, David A. Peribulbar and retrobulbar combined anesthesia for vitreoretinal surgery using ropivacaine. Eur J Ophthalmol. 2006;16(2):295.
- ↑ Jump up to: 28.0 28.1 28.2 Rey A, Jurgens I, Maseras X, Dyrda A, Pera P, Morilla A. Visual outcome and complications of cataract extraction after pars plana vitrectomy. Clin Ophthalmol. 2018; 12:989–994. doi:10.2147/OPTH.S161223.
- ↑ Sneed S, Parrish RK, Mandelbaum S, O'Grady G. Technical Problems of Extracapsular Cataract Extractions After Vitrectomy. Arch Ophthalmol. 1986;104(8):1126–1127. doi:10.1001/archopht.1986.01050200032025
- ↑ Jump up to: 30.0 30.1 30.2 Wang EF, Worsley A, Polkinghorne PJ. Comparative study of femtosecond laser-assisted cataract surgery and conventional phacoemulsification in vitrectomized eyes. Clin Exp Ophthalmol. 2018;46(6):624–629. doi:10.1111/ceo.13133.
- ↑ Anisimova N, Malyugin B, Arbisser LB, Sobolev N. Femtosecond laser-assisted cataract surgery in vitrectomized eye with posterior chamber phakic intraocular lens. Digit J Ophthalmol. 2017; 23(2):43–44. doi:10.5693/djo.02.2017.03.001.
- ↑ Jump up to: 32.0 32.1 32.2 Braunstein RE, Airiani S. Cataract surgery results after pars plana vitrectomy. Curr Opin Ophthalmol. 2003;14(3):150–154. doi:10.1097/00055735-200306000-00007.
- ↑ Jump up to: 33.0 33.1 33.2 De Maria M, Iannetta D, Cimino L, Coassin M, Fontana L.<p>Measuring anterior chamber inflammation after cataract surgery: a review of the literature focusing on the correlation with cystoid macular edema. Clin Ophthalmol. 2020; 14:41–52. doi:10.2147/OPTH.S237405.
- ↑ Jump up to: 34.0 34.1 34.2 34.3 Pardo-Munoz A, Muriel-Herrero A, Abraira V, Muriel A, Munoz-Negrete FJ, Murube J. Phacoemulsification in previously vitrectomized patients: an analysis of the surgical results in 100 eyes as well as the factors contributing to the cataract formation. Eur J Ophthalmol. 2006;16(1):52–59. doi:10.1177/112067210601600110.
- ↑ Bryan EA, Cruz-Inigo YJ, Brems RN, Bryan JS Acute macular edema with serous retinal detachment after cataract surgery in a vitrectomized eye: a case report. Retin Cases Brief Rep. 2019.
- ↑ Zuberbuhler B, Seyedian M, Tuft S. Phacoemulsification in eyes with extreme axial myopia. J Cataract Refract Surg. 2009;35(2):335-40.
- ↑ Chang MA, Parides MK, Chang S, Braunstein RE. Outcome of phacoemulsification after pars plana vitrectomy. Ophthalmology. 2002;109(5):948–954. doi:10.1016/S0161-6420(01)01010-7.